Ceraxon tablets: composition and action
Ceraxon is sold in several forms:
- injections;
- pills;
- solution for systemic use.
The tablets contain the main active ingredient – citicoline sodium. In addition to this, the composition includes:
- talc;
- castor oil;
- magnesium stearate.
Tablets are packed in 5 pieces in a blister, each box contains 4 blisters. The solution intended for drinking contains the same basic substance - citicoline, and along with it glycerin, sorbidol, sodium citrate, sodium saccharinate, strawberry flavoring. This composition is packaged in unpainted glass bottles with plastic caps. The volume of one bottle is 30 ml. It is convenient to dose using the syringe included in each package.
The drug for administration into a vein is packaged in glass ampoules, with a break line marked. One cardboard package can contain 3 or 5 pieces. Ceraxon mainly acts on brain cells, strengthening membranes, improving the functions of ion exchange pumps of the nervous system, which is due to the production of phospholipids.
In case of severe swelling, the action of the drug relieves pressure at the site of the lesion, which returns the cognitive functions of the brain. In addition, the ability to concentrate is restored and memory improves. If Cerakson is applied promptly at the first symptoms of a stroke, it will be possible to minimize the area of spread of necrotic tissue.
Citicoline is one of the constant elements of the human body, which does not make it possible to evaluate its pharmacokinetics. The substance entering through the gastrointestinal tract is absorbed almost completely. As a rule, 1-2% of the total ingested volume remains unabsorbed. Citicoline is excreted through the kidneys in the urine.
As a result of hepatic metabolism processes, two substances are formed: cytidine and choline. Citicoline is first concentrated in the structures and tissues of the brain. At the same time, fractions of the resulting choline pass into phospholipids, and cytidine derivatives into the composition of nucleic acids.
Comparison of the effectiveness of Ceraxon and Citicoline
The effectiveness of Ceraxon is quite similar to Citicoline - this means that the ability of the drug to provide the maximum possible effect is similar.
For example, if the therapeutic effect of Ceraxon is more pronounced, then using Citicoline even in large doses will not achieve this effect.
Also, the speed of therapy - an indicator of the speed of therapeutic action - is approximately the same for Ceraxon and Citicoline. And bioavailability, that is, the amount of a drug reaching its site of action in the body, is similar. The higher the bioavailability, the less it will be lost during absorption and use by the body.
When is Cerakson prescribed?
Treatment with Ceraxon is indicated in the following cases:
- first 4 hours during primary stroke;
- for rehabilitation after a stroke;
- emergency assistance and to restore the condition impaired as a result of severe head injury;
- cerebrovascular diseases that reduce cognitive function and provoke behavioral changes.
If the patient was administered Ceraxon in the first hours after a traumatic brain injury, the duration of post-traumatic coma will be less than without the help of the drug. In addition, there is a high probability of reducing the severity of neurological abnormalities typical of this type of injury.
If chronic hypoxia has become the reason for the predominance of lack of initiative in a person’s behavior, complete inability in matters of self-care, the drug effectively affects certain areas of the brain, restoring cognitive functions.
Comparison of addiction between Ceraxon and Citicoline
Like safety, addiction also involves many factors that must be considered when evaluating a drug.
So, the totality of the values of such parameters as “o syndrome” in Cerakson is quite similar to the similar values in Citicoline. Withdrawal syndrome is a pathological condition that occurs after the cessation of intake of addictive or dependent substances into the body. And resistance is understood as initial immunity to a drug; in this it differs from addiction, when immunity to a drug develops over a certain period of time. The presence of resistance can only be stated if an attempt has been made to increase the dose of the drug to the maximum possible. At the same time, in Cerakson the meaning of “syndrome” is quite small, however, the same as in Citicoline.
Ceraxon 1000: instructions
Intravenous administration of the drug Ceraxon 1000 should be carried out slowly and evenly, avoiding the creation of a strong stream of flow. When administering drips, the lamb should be in the middle position, which provides up to 60 drops per minute. Jet injection, including into an elastic band, is carried out by applying gentle pressure on the syringe piston. The effectiveness of the drug directly depends on the time of treatment. The sooner the patient was admitted to the care of physicians, and the earlier drug treatment was started, the more positive the result will be.
Ceraxon 1000 is a classic dosage for the treatment of stroke of various etiologies and to minimize the consequences of traumatic brain injuries, which is administered every 12 hours for 6 weeks. After which, if the patient has not lost the swallowing reflex, intravenous administration is replaced by systemic administration through tablets.
If treatment involves taking the solution orally, it must be diluted in 100 ml of drinking water. Drink the medicine with meals. Children may be prescribed the drug in cases of damage to the central nervous system. The dosage for the child is prescribed by the attending physician, depending on his weight, age and condition.
The problem of the coexistence of reproduced (generic or generic) and original medicines (medicines) is very relevant for various countries of the world, incl. and Russia. Discussions are constantly taking place on the comparability of the effectiveness and safety of generic and original drugs. Various arguments and arguments are given in favor of the preferential use of both one and the other. Unfortunately, in Russia, not all doctors still clearly understand the difference between original and generic drugs, how they are created, and what advantages and/or weaknesses they have.
Let us give a definition of the concepts of original and reproduced (generic) drugs. These definitions are formulated in various paragraphs of the Federal Law of the Russian Federation “On the Circulation of Medicines” No. 61 of 04/12/2010. This law today is the fundamental regulatory legal act for all processes related to the study, registration and use of drugs in Russia.
The law defines that an “original drug” is a drug containing a pharmaceutical substance obtained for the first time or a new combination of pharmaceutical substances, the effectiveness and safety of which have been confirmed by the results of preclinical studies of the drug and clinical trials of drugs. “Reproduced medicinal product” is a medicine containing a similar pharmaceutical substance or a combination of the same pharmaceutical substances in the same dosage form as the original medicine, which came into circulation after the original medicine went into circulation.
Based on these definitions, let’s remember how doctors’ arsenal includes new medicines, which are called original? There are a relatively small number of large pharmaceutical companies in the world engaged in the development of new (innovative, original) drugs. This is due to the fact that the process of creating a new drug is very labor-intensive, high-tech, expensive and lengthy. From the moment the idea of creating a new drug arises to the moment it is registered and begins widespread clinical use, many sequential events occur. It all starts with the search for new pharmacologically active compounds that can solve the problem set by researchers of effectively influencing the designated pharmacological target. The selection of the most promising molecules and assessment of their potential are carried out using a large number of in silico, in vitro and in vivo models. A procedure for assessing the acute, chronic and specific toxicity of the most promising molecules is mandatory. The pharmacokinetic parameters of the new drug are being studied in animals. If extensive preclinical studies are successful, the most successful molecule of a potential drug, enclosed in one or another dosage form, becomes the object of long-term clinical trials in accordance with all international requirements and standards of good clinical practice (GCP). This process includes three sequential phases of clinical trials (CTs). If a drug successfully passes trials in three phases of a clinical trial, it receives a marketing authorization and begins to be widely used in medical practice to treat patients [5–7].
Phase I clinical trials typically involve 20 to 100 healthy volunteers. Sometimes the high toxicity of a drug (for example, drugs for the treatment of cancer or HIV infection) makes such a study of healthy volunteers unethical. In this case, it is carried out with the participation of patients suffering from the corresponding disease. The purpose of the Phase I clinical trial is to establish the tolerability, pharmacokinetic (absorption, distribution, metabolism, excretion) and pharmacodynamic parameters of the drug, as well as to provide a preliminary assessment of its safety.
Having assessed the pharmacokinetics and pharmacodynamics, as well as the preliminary safety of the drug during a phase I clinical trial, the company developing the original drug initiates phase II studies on a patient population of 100–500 people. Phase II clinical trials are usually performed on a patient population selected according to strict criteria. An important goal of this study is to obtain evidence of the effectiveness of the new drug, to select its optimal dose and dosage regimen for phase III CT. The doses of the drug that patients receive in phase II studies are typically lower than the highest doses given to participants in phase I.
The Phase III CT is a randomized controlled multicenter trial involving a patient population of 300–3000 or more depending on the disease. It is designed to demonstrate the safety and effectiveness of a drug for a specific indication in a specific patient population.
Phase III clinical trials can study the dose-dependent effect of the drug. Having confirmed the effectiveness and safety of the drug in phase III studies, the company forms the so-called. registration dossier of the drug, which describes the methodology and results of preclinical and clinical studies, production features, composition, shelf life. The totality of this very voluminous information is submitted to the authorized health authority that carries out the registration.
Phase IV studies are conducted after the registration of the original drug. As part of phase IV, the clinical trials necessary to optimize its use are carried out. An important task of phase IV is to collect additional information about the safety of the drug in a sufficiently large population of patients over a long period of time. The goals of phase IV clinical trials may include assessment of parameters such as treatment duration, interaction with other drugs or food products, comparative analysis of standard courses of treatment, analysis of drug use in patients of different age groups, economic indicators of treatment and long-term results of therapy. In observational (non-interventional) CTs, after registration, information is collected on how the drug is used by doctors in their daily clinical practice, which makes it possible to judge its effectiveness and safety in “real life” conditions. If rare but serious adverse events are detected during phase IV clinical trials or post-marketing observational studies, the drug may be withdrawn from the market or its use may be limited.
It is believed that on average, all stages of the development of an original drug take up to 12–15 years, and costs can significantly exceed $1 billion. It is no coincidence that in the last decade there has been a concentration of the pharmaceutical industry, a merger of the largest pharmaceutical companies, which allows them to bear the enormous costs of research and the introduction of new drugs. The company that developed the original drug receives a patent for it. The validity period of the latter provides the company with the right to exclusive production and distribution (sale) of the drug in the first years after its registration. This should offset the cost of developing the drug and also enable the company to make a profit. Thus, the undoubted advantage of the original drugs is that they are new, high-quality modern drugs, studied on thousands of patients in compliance with all GCP requirements; the effectiveness and safety of these drugs have actually been proven both at the stages of clinical trials before registration and in large clinical trials after registration . A relative disadvantage of original drugs is the fact that they can be quite expensive. However, it should be taken into account that, according to pharmacoeconomic studies, the final cost of treatment when using a new, expensive, high-quality original drug can often be less than when using alternative drugs. This is achieved by reducing the patient’s time in the hospital, improving his ability to work, reducing the number of adverse events, hospitalizations, incl. repeated
How do generic drugs appear in the arsenal of doctors? These drugs go through a completely different path from the synthesis stage to the moment of registration and the beginning of widespread clinical use. After the expiration of the patent for the original medicine, it becomes an “international property”, i.e. the active principle of the drug and dosage forms containing it can be legally reproduced by other pharmaceutical companies (generics). The main question that is the subject of heated debate is: how interchangeable are original and generic drugs? It is absolutely clear that generic drugs do not undergo the multi-stage procedure for studying the effectiveness and safety for animals and humans, which was described above. What is offered in return? In various countries of the world, incl. in Russia, various types of equivalence of original and generic drugs are being studied. There is a concept of “pharmaceutical equivalence”. Pharmaceutical equivalents (pharmaceutically equivalent drugs) are drugs that contain identical pharmaceutical substances in the same doses (concentrations), dosage forms and chemical modifications (for example, identical salts or esters), meet established standards of identity, strength, quality and purity. Pharmaceutical equivalents may differ in appearance, notches on tablets, packaging, composition of excipients (including dyes, flavors and preservatives), expiration date, etc. Pharmaceutical equivalence does not guarantee pharmacokinetic bioequivalence, and therefore does not replace the assessment of bioequivalence - the main study underlying the registration of a drug that has a systemic bioavailability parameter.
The Federal Law “On the Circulation of Medicines” defines that “a study of the bioequivalence of a medicinal product is a type of CT of a medicinal product, which is carried out to determine the rate of absorption and excretion of a pharmaceutical substance, the amount of a pharmaceutical substance reaching the systemic bloodstream, the results of which allow us to draw a conclusion about the bioequivalence of the reproduced medicinal product in a certain dosage form and dosage corresponding to the original medicinal product.” It is generally accepted that bioequivalent drugs are therapeutically equivalent and, therefore, interchangeable. Assessment of the bioequivalence of a generic to the original drug is based on a pharmacokinetic study. The results of the study allow us to compare the bioavailability of original and generic drugs, i.e. comparison of indicators characterizing the rate and extent to which the active pharmaceutical substance is absorbed from the drug and enters the systemic circulation, which implies entry into the site of action [4].
A pharmacokinetic bioequivalence study is carried out in two stages using a crossover randomized trial design of two drugs in a limited number of healthy volunteers (from 18 to 50 people). The subjects are administered single doses of generic and original drugs and the level of the drug in the blood plasma is measured at specified time intervals. At the same time, at the first stage of the study, half of the test volunteers randomly receive the original drug, the other half - the generic one. At the second stage of the study, the opposite order of drug administration is used. As part of the statistical analysis of the results of bioequivalence studies, mean values and 90% confidence intervals are calculated for a number of pharmacokinetic parameters (Cmax, TCmax and AUC). For each indicator, the ratio of the corresponding values of generic and original drugs is calculated. Based on the results of the bioequivalence study, a detailed report and other documentation are drawn up in accordance with existing requirements. These documents form the basis of the registration dossier of the drug. Further, the process of registration of the reproduced drug occurs within the framework of the procedures provided for by the law of the Russian Federation “On the Circulation of Medicines”. The main and only advantage of reproduced drugs over original ones is their lower cost. The low price of a generic drug is explained by the fact that its creation and registration do not require such colossal costs as when creating an original drug. However, it should be emphasized once again that these drugs do not undergo actual efficacy testing in patients, but are subjected only to comparative pharmacokinetic testing with the original drugs.
The most reliable criterion for comparative assessment of the effectiveness of generic and original drugs remains the assessment of therapeutic equivalence. A therapeutic equivalence study is a type of clinical trial, which is carried out to identify the same properties of drugs of a certain dosage form, as well as the presence of the same indicators of safety and effectiveness of drugs, the same clinical effects when used. In the Russian Federation, this study is not mandatory for generic drugs and is almost never carried out. At the same time, in the USA, generic drugs that have passed therapeutic equivalence studies are included in the so-called. FDA Orange Book. Accordingly, generic drugs are divided into category “A” (that have passed therapeutic equivalence studies) and category “B” (that have not passed these studies). Category A drugs are preferable for use.
A therapeutic equivalence study involves 70–150 patients. It is advisable that the study be conducted in two or three clinical centers. The nosological homogeneity of the study group of patients, the adequacy of the method for assessing therapeutic effectiveness and statistics are important. The number of patients included in the study is determined by its design, the potential spread of indicator values that reflect the effects of the drugs being compared during the study. The “non-inferiority” hypothesis is tested. The main criterion for including patients in a therapeutic equivalence study is the indication for use, regulated in the instructions for medical use of the original drug, i.e. the indication for which the original and generic drugs will be compared. Both bioequivalent and therapeutically equivalent drugs may differ in tablet form, notches, packaging, excipients (including colors, flavors and preservatives), expiration date, storage conditions and stability under adverse storage conditions. It should be borne in mind that if the original drug taken by the patient is replaced with a generic one with the above differences, patients may become disoriented due to a different shape or color of the tablets, or the inability to measure the required dose when using part of the tablet if there are no notches. There may be a taste aversion to certain drugs due to the filler, or the development of an allergic reaction to the dye or preservative. If such differences are important to the treatment of a particular patient, the treating physician should prefer to dispense a drug with a specific brand name on a medical necessity basis. This can be done by decision of the medical commission of the medical organization.
Ceraxon® (citicoline) is a striking example of an original drug used in neurology. The presence of a full range of preclinical (in vitro and in vivo on laboratory animals) and clinical (on healthy volunteers and various patient populations) studies is the main difference between the original citicoline (Ceraxon®) and its generic versions. Details of the mechanism of action of citicoline, pharmacokinetic parameters, optimal doses and modes of administration, interaction with other drugs in complex drug therapy have been studied in depth and in detail using the original drug Ceraxon®. All this data is presented in detail in the registration dossier of the drug. After the study of Cerakson® according to all the rules of preclinical studies and GCP, it began to be widely used by patients with various pathologies. Citicoline (cytidine-5-diphosphocholine) is an organic substance of a group of nucleotides, biomolecules that play an important role in cellular metabolism. Citicoline can be endogenous or exogenous. The endogenous formation of citicoline is a step in the synthesis of phosphatidylcholine from choline. Citicoline is an essential precursor of phosphatidylcholine (lecithin), the main phospholipid of all cell membranes, including neuronal ones. Choline also takes part in the synthesis of acetylcholine, and citicoline acts as a donor of choline in the processes of its synthesis. Exogenous citicoline allows you to save choline reserves in the body, inhibit the breakdown of membrane phospholipids, and improve the transmission of nerve impulses [3, 23, 26, 27]. When entering the gastrointestinal tract, exogenous citicoline is hydrolyzed in the small intestine. As a result of hydrolysis, choline and cytidine are formed in the intestinal wall and in the liver (the latter is then transformed into uridine). After absorption, they enter the systemic circulation, participate in various biosynthetic processes and penetrate the blood-brain barrier into the brain, where citicoline is resynthesized from choline and cytidine. Citicoline resynthesized in the brain activates the biosynthesis of phosphatidylcholine and prevents its catabolism from neuronal membranes. It maintains normal levels of cardiolipin (a major component of mitochondrial membranes) and sphingomyelin. Citicoline inhibits the synthesis of phospholipase A2, reduces the accumulation of free fatty acids, enhances the activity of antioxidant systems (stimulates the synthesis of glutathione), prevents the processes of oxidative stress and apoptosis, has a positive effect on cholinergic transmission, stimulating the formation of acetylcholine, and also modulates dopamine and glutamatergic neurotransmission. All these effects contribute to the activation of energy processes in neurons, normalize tissue respiration processes, and lead to a decrease in apoptotic cell death [10]. For more than 40 years, Ceraxon® (citicoline) has been used in the USA, Japan and European countries for the treatment of cerebral stroke (ischemic and hemorrhagic), traumatic brain injury (TBI), and cognitive impairment. Citicoline today is one of the few neuroprotectors with high evidence of effectiveness in studies [2, 8, 11].
All large CTs with a high level of evidence were conducted with the original citicoline drug Ceraxon®. Thanks to a wealth of evidence-based research, Ceraxon® (citicoline) is the only neuroprotector included in the 2008 European guidelines for the treatment of stroke [20]. The first double-blind, multicenter, placebo-controlled study to study the effect of Ceraxon® in cerebral infarction was conducted in Japan in 1988 [28].
It demonstrated that Ceraxon® is effective for the treatment of acute stroke. Similar results were obtained later in research centers in other countries [18]. In 1997, the first double-blind, randomized, multicenter clinical trial using oral Ceraxon® (citicoline) for the treatment of acute ischemic stroke was launched in the United States, assessing the effectiveness of different doses of the drug [14]. The results of the study indicated the clinical effectiveness of oral administration of Ceraxon®, and the dose of 500 mg was the most effective and justified. A large study examining the effectiveness of Ceraxon® (citicoline) in ischemic stroke, ECCO-2000, was conducted in the USA in 2000–2001. it included 899 patients with moderate to severe stroke [15]. The ECCO study assessed the effect of Ceraxon® on lesion volume in cerebral infarction using the latest neuroimaging techniques. In a fairly large number of patients treated with Ceraxon® (citicoline), a decrease in lesion volume was noted, which significantly correlated with clinical improvement. A number of reviews have also drawn conclusions about the feasibility of prescribing Ceraxon® for stroke, its safety and good tolerability by patients [16, 26]. The multicenter, randomized, placebo-controlled ICTUS trial involving 2298 patients was conducted in 59 centers in Spain, Germany and Portugal. The study assessed the effectiveness of treatment with Ceraxon® (citicoline) for acute moderate and severe ischemic stroke [13]. The effect of the drug was pronounced in subgroups of patients over 70 years of age with moderate stroke severity, as well as those who did not receive concomitant treatment with tissue plasminogen activator.
Many studies have been conducted that have established the effectiveness of Ceraxon® (citicoline) in the treatment of cognitive disorders of neurodegenerative, involutional and vascular origin [9, 19, 24, 25]. In 2005, a Cochrane review was published on the use of citicoline for the correction of cognitive and behavioral disorders in chronic cerebrovascular pathology in the elderly, which concluded that this drug is useful for the correction of such disorders [17]. Extensive clinical experience has been accumulated in the use of citicoline in the treatment of TBI and its consequences. So, starting from the 1980s. A large number of clinical studies, including double-blind studies, have been conducted on TBI patients with various impairments in level of consciousness. The results of these studies, summarized in a meta-analysis, indicate that the use of Ceraxon® for TBI is clinically and pathogenetically justified, the drug accelerates the leveling of cerebral edema and the restoration of both consciousness and neurological functions, helps reduce the duration of hospitalization and improve rehabilitation results [ 22].
The study of the original Ceraxon® citicoline continues to this day, almost 50 years after its registration. Experimental, as well as randomized controlled and open clinical studies are being conducted to evaluate the effectiveness of the drug in people with various pathologies, including ischemic stroke, cognitive impairment of various origins, methamphetamine and alcohol addiction.
There are quite a lot of generic drugs containing citicoline on the pharmaceutical market today. However, all of them, unlike the original drug Ceraxon®, did not undergo a large number of clinical trials for various types of neurological pathology before and after registration. Their registration relied on the traditional equivalence studies described above. It is very important that in the case of citicoline, a correct bioequivalence study is quite difficult to conduct, since it is a natural compound found in the human body. In such a study, it is extremely difficult to differentiate exogenous and endogenous citicoline and determine their quantity.
It should also be noted that in the case of Cerakson® and its generics, the isomeric composition of the molecules included in the active substance of the drug is important for the implementation of the therapeutic effect. It is known that the contribution of the phenomenon of optical isomerism to the therapeutic non-equivalence of generics is sometimes very significant [1, 12, 21]. The citicoline molecule is capable of forming more than 4 stereoisomers with different biological activities. Depending on the method of synthesis, different manufacturers produce a mixture with different ratios of citicoline isomers, which can affect the pharmacological activity of the drugs they produce. Differences in the synthesis method can also lead to the production of a product with a higher content of degradation products and technological impurities than in the original preparation [29].
There is no doubt that the confrontation and at the same time the coexistence of original and generic drugs in the pharmaceutical market will continue. It is dialectical and inevitable. However, there is also no doubt that a doctor’s choice of a drug for drug therapy should be based on confidence in the proven effectiveness and safety of the drug. This is especially true for drugs whose pharmacodynamics and pharmacokinetics are closely intertwined with natural biochemical processes in the body, due to which evidence of pharmacokinetic equivalence for them is not absolutely convincing. The use of the original Ceraxon® citicoline allows the doctor to be confident in the full implementation of the pharmacodynamic effects of the drug, and therefore in the potentially successful treatment of a wide range of neurological diseases for which it is indicated and used.
Contraindications for use
If, as a result of diagnosing the patient’s condition, the following features are revealed, Cerakson is not prescribed:
- individual intolerance to one of the components of the drug;
- tone of the autonomic nervous system, in its parasympathetic part;
- hereditary fructose intolerance.
Adverse reactions during treatment with Cerakson are extremely rare, among them are:
- coordination problems;
- pain in the occipital region;
- diarrhea;
- appetite disorders;
- insomnia;
- rash.
Sometimes short-term changes in blood pressure are observed, as well as loss of sensation, mainly in the arm and leg on the paralyzed side, if the patient has suffered a stroke.
If any of these reactions occur, you should discuss changing the course of therapy with your doctor.
Comparison of side effects of Ceraxon and Citicoline
Side effects or adverse events are any adverse medical event that occurs in a subject after administration of a drug.
Ceraxon has almost the same level of adverse events as Citicoline. They both have few side effects. This implies that the frequency of their occurrence is low, that is, the indicator of how many cases of an undesirable effect of treatment are possible and registered is low. The undesirable effect on the body, the strength of influence and the toxic effect of Ceraxon are similar to Citicoline: how quickly the body recovers after taking it and whether it recovers at all.
special instructions
Treatment with Cerakson is often prescribed to premature babies and newborns with brain tissue injuries received during childbirth. The treatment regimen is determined by the attending physician and depends on the Apgar assessment of the child’s condition. Storing the medicine in the refrigerator may cause crystals to form in the solution. It will take at least two months at room temperature to dissolve them. Despite the fact that this does not reduce the quality of the active substance, the drug should still be stored at room temperature not exceeding +30 C.
The empty packaging of the drug must be used within three years from the date of release.
Comparison of the safety of Ceraxon and Citicoline
The safety of a drug includes many factors.
At the same time, in Ceraxon it is quite similar to Citicoline. It is important where the drug is metabolized: drugs are excreted from the body either unchanged or in the form of products of their biochemical transformations. Metabolism occurs spontaneously, but most often involves major organs such as the liver, kidneys, lungs, skin, brain and others. When assessing the metabolism of Ceraxon, as well as Citicoline, we look at which organ is the metabolizing organ and how critical the effect on it is.
The risk-benefit ratio is when the prescription of a drug is undesirable, but justified under certain conditions and circumstances, with the obligatory observance of caution in use. At the same time, Ceraxon does not have any risks when used, just like Citicoline.
Also, when calculating safety, it is taken into account whether only allergic reactions occur or possible dysfunction of the main organs. In other matters, as well as the reversibility of the consequences of using Ceraxon and Citicoline.
Medical Internet conferences
Chronic cerebral ischemia is a multifactorial disease, occurs mainly in older age groups, is characterized by clinical and morphological heterogeneity, and is characterized by a progressive course. Modern angioneurology has achieved great success in the treatment of patients with chronic cerebrovascular insufficiency. The use of drugs that help optimize metabolism, stabilize the cell membrane, and influence the processes of neuroplasticity is justified from a pathogenetic point of view.
Purpose of the study. Studying the effectiveness of the drug "Ceraxon" in patients with chronic cerebral ischemia of stage II of mixed origin.
Materials and methods. A comprehensive clinical and instrumental examination was carried out on 86 patients (47 women and 39 men) who were treated in the neurological department of the Municipal Clinical Hospital No. 9, Saratov, with chronic cerebral ischemia (CHI) stage 2 of mixed origin with focal neurological symptoms and moderate cognitive impairment . The average age of the patients was 68+1.2.
The patients were divided into 2 groups. The main group (MG) included patients (55 people) in whose treatment, in addition to basic therapy, the drug “Ceraxon” was used at a daily dose of 1000 mg intravenously in 150 ml of 0.9% saline solution for 10 days. The comparison group (CG) consisted of patients (31 people) who received standard therapy (nootropics, antioxidants, antiplatelet agents, physiotherapeutic treatment, therapeutic exercises). The groups were comparable by gender, age, and nature of vascular pathology.
The diagnosis was established based on the results of a neurological and neuropsychological examination, laboratory and instrumental data (ultrasound of the head and neck, nuclear magnetic resonance imaging) research methods.
Before and after the end of the course of treatment, complaints and neurological status of patients were analyzed, and neuropsychological indicators were assessed. Screening assessment of cognitive functions was carried out using the MMSE scale. Memory was assessed using the method of memorizing 10 words and an associative memory test, attention was studied using the Kraepelin counting method, spatial and dynamic praxis were studied, the level of anxiety was determined using the Spielberger-Hanin scale.
Results and discussion. Analysis of the structure of complaints revealed the presence of general cerebral symptoms, such as headache, dizziness, general weakness, as well as sleep disturbance and emotional lability. The neurological status was represented by pyramidal, cerebellar, extrapyramidal, and pseudobulbar syndromes. Before starting treatment, a neuropsychological study revealed a moderate cognitive deficit in all patients in the form of a disorder of mnestic function, attention deficit, decreased cognitive activity, and rapid exhaustion. The majority of patients had anxiety of varying degrees of severity.
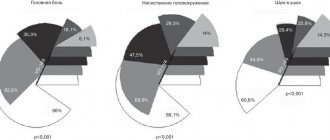
After the course of treatment, the subjective well-being of patients both in the MG and GS significantly improved. Positive dynamics in the form of a decrease in headaches, dizziness, improved sleep, and normalization of mood prevailed in the MG. As a result of the therapy, the severity of focal neurological symptoms decreased, especially in relation to cerebellar and pseudobulbar syndromes. In the OG, the cerebellar syndrome regressed in 69.1% of patients, in the GS – in 48.4% of cases.
After the course of treatment, activation of the functions of perception and memory of information was noted, and the number of words during reproduction increased. Activation of the mnestic function was observed in the OG before treatment - 7.2 + 0.2, after - 9.0 + 0.1*, in the GS before - 7.1 + 0.2 after - 8.5 + 0.2; stability and concentration of attention increased in the MG before treatment - 65.5+1.8, after - 52.6+1.8*, in the GS before - 64.9+2.3, after - 58.1+2.2 ; dynamic praxis indicators improved in the MG before treatment - 3.8+0.3, after - 4.6+0.2*, in the GS before - 3.8+0.1, after - 4.4+0.2* , spatial praxis in the OG before treatment - 10.2+0.2, after -12.8+0.2*, in the GS before - 9.2+0.2, after - 10.7+0.2* ( * - reliability criterion in comparison with indicators before treatment < 0.05). Positive dynamics were recorded during a repeated study of the level of anxiety: OG before treatment – 60.2+6.9, after – 51.6+8.1*, in GS before – 60.6+9.9, after – 54.9+ 10.1 (* - reliability criterion in comparison with indicators before treatment < 0.05).
Conclusions. This study confirms that the use of the drug "Ceraxon" in patients with chronic cerebral ischemia stage II leads to regression of neurological syndromes and improvement of the cognitive sphere. The use of this drug allows you to achieve the best therapeutic result and contributes to effective medical rehabilitation.
Comparison of ease of use of Ceraxon and Citicoline
This includes dose selection taking into account various conditions and frequency of doses. At the same time, it is important not to forget about the release form of the drug; it is also important to take it into account when making an assessment.
The ease of use of Ceraxon is approximately the same as that of Citicoline. However, they are not convenient enough to use.
The drug ratings were compiled by experienced pharmacists who studied international research. The report is generated automatically.
Last update date: 2020-12-04 13:49:30