Multiple sclerosis is an autoimmune disease of the central and peripheral nervous system, which is characterized by the disseminated formation of demyelinating pathological foci with the occurrence of symptoms in accordance with their localization. To this day, there are many questions regarding this pathology. What triggers an autoimmune process? What pathogenetic mechanisms underlie the disease? How to stop this process? Thanks to numerous scientific studies, it was possible to study many aspects of this pathology.
Previously, it was believed that multiple sclerosis mainly affects middle-aged people (30-50 years old). However, world statistics indicate an increase in the number of cases of demyelinating diseases among children. The proportion of multiple sclerosis in the age category from 0 to 16 years is about 1.2–6% of all patients.
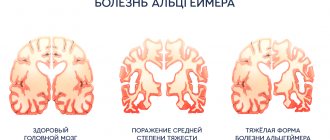
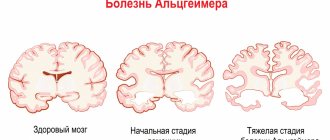
The main pathogenetic link in the development of multiple sclerosis in children is a dysfunction of the immune system. As a result of exposure to trigger factors, a cascade of autoimmune reactions is launched. The synthesized T lymphocytes penetrate the blood-brain barrier and selectively destroy the myelin sheath of neurons and their axons. Fibrous plaques form in place of normal nerve tissue. The number of demyelinating foci and their size are determined by the activity of the pathological process.
In multiple sclerosis, not only the nerve cells of the central nervous system, but also the peripheral ones, succumb to autoimmune aggression. This statement was a real discovery in neurology. If just a few years ago disorders of the peripheral nervous system excluded the diagnosis of multiple sclerosis, today such variants of the course of the disease have been scientifically confirmed.
Causes of multiple sclerosis
The main pathogenetic mechanism of this disease is the sensitization of T-lymphocytes by myelin antigens and the activation of autoimmune reactions against their own nerve cells. It has not yet been possible to reliably establish the reasons for this process. There are several predisposing factors that can initiate a cascade of pathological immune response. These include:
- Infectious agents . According to this hypothesis, some viruses (adenoviruses, herpes viruses, mumps, measles) can trigger the synthesis of antimyelin antibodies. The genetic material of viruses is represented by a chain of nucleic acids. Some of its parts coincide with myelin proteins. Thus, antigen-presenting cells (APCs) perceive their own genetic material as foreign. After the exchange of information between APCs and T cells, an autoimmune reaction is launched against the myelin sheath of neurons and oligodendroglia, and inflammatory cytokines (interleukin-2, tumor necrosis factor, interferon gamma) are synthesized in the pathological focus, which lead to destructive processes.
- Heredity . According to statistics, the risk of multiple sclerosis in a child whose family has had a case of this disease is higher than in the general population.
- Environmental factors . The results of some studies confirm the fact that multiple sclerosis is more common in residents of geographical zones remote from the equator. This is explained by the low amount of vitamin D in representatives of the Caucasian race compared to residents of equatorial regions.
- Vaccination of children against hepatitis B and stress factors are not reliable reasons for the activation of autoimmune aggression.
Viral infections
The influence of infectious agents is one of the fundamental factors in the development of MS, but until now the exact mechanism of influence has not been studied.
There is no evidence to suggest that MS is a direct consequence of any specific disease. At the same time, there is evidence that some infectious agents can serve as a trigger in the development of multiple sclerosis: retroviruses, measles, herpes, rubella, mumps, Epstein-Barr viruses. There is also evidence of the possible influence of staphylococcal infection [6, 7].
Prevalence rate:
Types of multiple sclerosis
This disease in children is classified according to the clinical course of the disease:
- Remitting-relapsing variant of the course . Episodes of increasing neurological deficit of the disease are followed by periods of partial or complete recovery. During an exacerbation, the neurological deficit increases gradually over several days to several weeks, exacerbation reaches a plateau phase, and then the impaired functions are gradually restored over several weeks or months. In some cases, the exacerbation reaches its maximum within a few minutes or hours. It should be noted that during this course of the disease, in some patients the symptoms that arise do not resolve completely, and the neurological deficit increases over the years.
- Primary progressive variant of the course . After the onset of the disease, gradual progression of the pathology is observed. There may be “bright gaps” in the clinical picture.
- Secondary progressive variant of the course . Against the background of alternating periods of remission and relapse, a secondary increase in the clinical manifestations of the disease is observed.
- Progressive course with periods of exacerbation . The increase in clinical signs of the disease begins with its first manifestations. The progressive course is accompanied by severe periods of exacerbation.
In 5-10% of patients with multiple sclerosis (MS), the first clinical signs of the disease appear in childhood and adolescence [1-5]. In such patients, drugs for the immunomodulatory treatment of MS are used as both the first and second line of choice [6–9]. However, the majority of drugs for the treatment of MS in the pediatric population are used in the “off-label” mode, since most of them, including MS course-modifying drugs (MS drugs), have undergone clinical trials only in the treatment of adult patients. In some countries, the use of immunomodulatory drugs for the treatment of MS is permitted only for people aged 12 years and older.
In light of the registration of the first tableted immunomodulatory drug for the treatment of MS (fingolimod was approved for use in the USA, Europe and Russia in 2010, in Canada and Australia in 2011), as well as on the threshold of registration of a number of other new injectable and tablet drugs, in Over the next few years, practicing neurologists will inevitably encounter contradictions in age recommendations for most medications, especially those that have not undergone formal testing in the pediatric population. Fortunately, recently, after these issues were raised with the European (EMA) and American (FDA) pharmaceutical agencies, plans were developed to conduct studies of new pharmaceutical drugs for the treatment of MS in children and adolescents in order to clarify the range of age-related indications and contraindications.
When conducting therapeutic studies in the pediatric population of MS patients, it is necessary to take into account the possibility of including a relatively small number of patients, and this inevitably leaves a huge imprint on the study design and the reliability of statistical calculations. Moreover, pediatric researchers are faced with the decision-making characteristics of parents of patients, which imposes additional ethical obligations on researchers, significantly limiting clinical experience with the use of new medications compared to the adult population. All of the above was the reason for the creation of IPMSSG guidelines for the use of modern immunomodulatory drugs for the treatment of childhood MS, taking into account all the specific medical, ethical and social characteristics of childhood and adolescence, as well as taking into account the need and features of statistical analysis of clinical information.
The IPMSSG represents a group of specialists working in the field of MS: pediatric and adult neurologists, clinicians and researchers, representatives of MS societies and other related professional and public organizations who work to improve the quality of care for patients with MS and other demyelinating diseases in children and adolescents1. Team members reviewed available clinical experience with pediatric MS and published clinical trials using regional and international information resources in English, Italian, Spanish, French, German and Russian. The studies reviewed were classified according to the level of evidence (classes I–IV), according to the recommendations of the American Academy of Neurology [10]. The combined data was presented for discussion during a conference organized by the International MS Federation and the Canadian MS Society on September 26, 2010.
All existing recommendations, reflecting consensuses on the treatment of children and adolescents with MS, are devoted only to relapsing-remitting forms of the disease [11] and do not contain information on the treatment of primary progressive forms of MS in children, since this form of pediatric MS is extremely rare and studies in this area areas are limited to single clinical observations.
Features of the course of MS in children and adolescents
In general, MS with onset in childhood and adolescence is clinically identical to MS with onset in adulthood. However, some features of the pediatric variant of the disease must be taken into account. Children in the early stages of the disease are characterized by 2-3 times more frequent exacerbations than adult patients (the average annual frequency of exacerbations in children is 1.12-2.76 compared to 0.3-1.78 in adults [12]) . In approximately 1/3 of children, cognitive impairment is detected already in the early stages of the disease [13, 14]. Long-term clinical studies revealed progression of cognitive disorders over the next 2 years in 75% of patients [15]. The formation of persistent disability according to the EDSS (Expanded Disability Status Scale) scale takes longer in patients with pediatric onset of MS than in adult patients [2–18]. Nevertheless, the early onset of the disease leads to the fact that patients reach the stage of accumulation of persistent neurological disorders at an earlier age than adults. The hypothesis about the greater severity of the inflammatory demyelination process in children and adolescents with MS compared to adults is confirmed by comparative MRI studies [19, 20].
First-line drug therapy for pediatric MS patients
Use of interferons-β and glatiramer acetate in patients with relapsing-remitting MS
Interferons-β and glatiramer acetate have been used for more than 15 years as first-line drugs of choice in adult patients with MS, and are also widely used in the pediatric population based on the results of class I studies in adult patients. A number of pediatric studies have been published with class IV results of evidence regarding their safety and effectiveness in pediatric patients with MS [21–29] (Tables 1, 2, 3, 4). A survey of 42 clinicians in the United States showed that all of them had positive experiences using first-line DMTs to treat children and adolescents with MS [30]. A number of other publications [31–33] have also supported the use of affordable first-line DMTs in pediatrics as the standard for clinical practice1,2 [68].
Table 1. First-line treatment of pediatric MS with DMTs. Review of pediatric studies on intramuscular interferon β-1a (30 mcg once weekly)
Table 2. First-line treatment of pediatric MS DMTRS (interferon-β-1a - subcutaneous 22 or 44 μg)
Table 3. Treatment of R.S. pediatric first-line DMT (interferon-β-1b subcutaneous 250 mcg)
Table 4. First-line treatment of pediatric MS with DMT (glatiramer acetate)
Based on these facts, the IPMSSG recommends that all pediatric patients with definite relapsing-remitting MS [11] be prescribed interferon-β or glatiramer acetate3 as the standard first-line therapy.
The use of interferon-β and glatiramer acetate in patients with clinically isolated syndrome (CIS)
To date, there are no published data on the use of interferon-β and glatiramer acetate in children with first demyelinating episode, or CIS. At the same time, a number of research groups have found that the presence of clearly defined foci of periventricular localization or in the corpus callosum, as well as areas of local atrophy (so-called black holes) in T1 mode on MRI during the first demyelinating episode indicates a high risk of progression of the demyelinating process in the future [34-37]. There is evidence to suggest that in children and adolescents with CIS and an MRI pattern indicating a high risk of MS, administration of interferon β or glatiramer acetate may delay further exacerbations of the disease. In Russia, at the moment, the use of DMTs is annotated only in patients with definite MS.
Titration and dosage regimen
Only 2 published studies [26, 27] discuss different dosing regimens of interferon-β in children and adolescents with MS, but none of the available publications provide information on dose titration of interferon-β. In this case, one should take into account the results of therapeutic studies conducted in adult patients, which showed that there is no direct dependence of the effectiveness of interferons-β on body mass index. We recommend that if the drug is well tolerated, titrate interferons-β to the full dose recommended in adult neurological practice; in children under 12 years of age or with underweight, a dosage of 22 mcg of interferon-β-1a for subcutaneous administration can be used. Glytiramer acetate in adult practice begins to be used directly with a full dose; According to the available literature data, this drug is also not titrated in children and adolescents with MS.
Safety
Retrospective studies [21, 24–28] have shown a similar short-term safety profile in children and adults with interferon-β and glatiramer acetate. Studying the long-term safety profile of interferon-β and glatiramer acetate in children and adolescents is an important task for future research, regardless of the results obtained in the treatment of adult patients [10]. It must be remembered that the long-term safety profile of DMTs in the pediatric population includes the effect of therapy on the growth and puberty of the child and adolescent, which is currently unknown.
Inadequate response to first-line treatment for MS in children and adolescents
There is no complete consensus regarding the assessment of the adequacy of the response to DMT therapy in either children or adults, therefore drugs for the treatment of “aggressive” forms of the disease are used rather haphazardly [38–41]. Published recommendations for the treatment of MS in adult patients state that 1–2 exacerbations per year or no increase in the frequency of exacerbations [42–44], the appearance of 2 or more new T2 lesions on brain MRI, or 1 or more lesions actively accumulating gadolinium contrast , during the first year of treatment can be considered criteria for treatment failure [29]. According to a retrospective study of 258 pediatric patients with MS, 44% of them switched to another drug during the first 3 years of therapy due to insufficient therapeutic efficacy of the original drug [32]. These facts indicate a high frequency of drug changes due to their apparent ineffectiveness, and also emphasize the need to define clear criteria for the adequacy of response to therapy in children and adolescents with R.S. But such studies have not yet been conducted, and we have formulated working recommendations to determine the effectiveness of interferon-β and glatiramer acetate in pediatric patients with MS.
These recommendations certainly require further development, and they are subject to change as new reliable information becomes available.
The criteria for an inadequate response (ineffectiveness) to first-line DMT can be considered compliance with at least one of the following provisions: an increase or absence of a decrease in the frequency of exacerbations or the presence of new T2-hyperintense foci or T1 foci accumulating contrast agent on MRI compared with the study conducted before treatment; 2 or more significant clinical exacerbations or twice, with an interval of at least 1 month, active lesions identified on MRI over a period of 12 months or less, poor recovery after exacerbations (progression of disability by 1 point or more on the EDSS scale over 3 months. These criteria for treatment failure applicable under conditions of a minimum time of use of the drug in full dose before assessing the effectiveness of treatment for 6 months and full adherence to treatment.
The decision about the adequacy of the response to therapy should be made taking into account the individual characteristics of the patient and the course of the disease (symptoms and severity of exacerbations, localization of lesions, rate of disease progression, degree of cognitive impairment).
Data on the features of the subclinical course of MS in children and adolescents are also the prerogative of further research, therefore, when choosing therapy, it is preferable to focus mainly on the clinical manifestations of the disease. The decision to change therapy should take into account the possible side effects of the new drug.
Therapeutic tactics for inadequate response to first-line DMT treatment
In case of insufficient effectiveness of treatment, you can either replace the drug with another one related to the first line (another interferon-β or glatiramer acetate), or switch to a second-line drug of choice from those listed below. Studies conducted in adult MS patients treated with interferon-β have shown that the detection of neutralizing antibodies to interferon-β in serum is associated with a decrease in the therapeutic effect of the interferons themselves [45]. Such studies have not yet been conducted in a pediatric cohort of patients with MS, however, based on the biological similarity of the pathogenesis of the disease, it can be concluded that it is inappropriate to continue therapy with interferons-β in patients with clinical ineffectiveness of interferons and the presence of neutralizing antibodies in the serum.
In some patients with an inadequate response to first-line DMT, a 6-12-month course of combination therapy can be used in the form of adding monthly infusions of high doses of prednisolone salts to standard doses of interferon-β or glatiramer acetate. When prescribing such combination therapy, the spectrum of possible complications of corticosteroids, such as osteoporosis, arterial hypertension, hyperglycemia, cataracts and an increased risk of infectious processes, must be taken into account.
Studies conducted in small groups of sick children have shown that some require aggressive treatment with second-line drugs such as natalizumab [29, 39, 40] and cyclophosphamide [46]. One way or another, only long-term, large-scale studies with pharmacokinetic and pharmacodynamic monitoring of the effectiveness and safety of treatment will be able to clearly determine the place of second-line drugs in the treatment of pediatric MS [47–49].
The final decision to change an immunomodulatory drug in each specific clinical case should be made jointly by the attending physician, the patient and his family, based on a careful analysis of the potential benefits and risks of using all possible treatment options [50, 51].
Second-line drug therapy for sick children with MS
To date, a number of new drugs are still being tested in adult patients with R.S. Some of these drugs claim an important role in the future treatment of pediatric forms of MS, but, naturally, require special therapeutic studies in children and adolescents. However, at the moment it remains unclear which drugs need to be studied first. Let's dwell on individual therapeutic agents.
Natalizumab is approved for the treatment of relapsing-remitting forms of adult-onset MS based on class I studies in the US and Europe for patients with an inadequate response to first-line immunotherapy or with severe, rapidly progressive disease [52, 53]. In Russia, natalizumab is licensed for use from 18 years of age. The drug is well tolerated, but there is a risk of progressive multifocal leukoencephalopathy (PML) caused by the JCV virus and potential hepatotoxicity. This risk depends on the duration of natalizumab therapy. There are no data on the risk of PML in children.
There are several reports in the literature [29, 39] of cases of natalizumab use in children with MS. In one such study, of 269 pediatric patients with progressive MS followed in the United States, 15 (5.5%) received natalizumab as second or third line therapy. Only 2 of the children receiving natalizumab subsequently changed therapy: one experienced a hypersensitivity reaction, and one discontinued therapy due to side effects (gastrointestinal disturbances). Despite the large amount of literature regarding PML [49–51], no cases of PML associated with pediatric patients have been reported to date [54, 56]. Since the risk of PML increases with the duration of natalizumab use, this has led to a limitation of the total duration of continuous treatment to 2 years in children. For patients with a good response to therapy over a 24-month period, de-escalation to first-line therapy is carried out, as suggested for adults, with a preliminary wash-out period of 3 months (an individual decision can be made to reduce this period) [57 ].
Mitoxantrone is effective in reducing relapses and disability in relapsing-remitting and secondary progressive MS in adults. It was approved by the US Federal Drug Administration (FDA) for the treatment of active MS in adults in 2000. However, mitoxantrone is associated with an increased risk of severe complications such as leukemia, cardiomyopathy, infections and infertility. In addition, its side effects such as nausea, vomiting and alopecia significantly limit the use of this drug [58, 59]. All of this has led to a decline in its use in adults with MS. The IPMSSG does not recommend the use of mitoxantrone in children. Nevertheless, specialists from a number of countries, including Russia and Ukraine, have positive experience in using mitoxantrone in pediatric patients with aggressive MS [57].
Fingolimod has been approved for use in adult MS patients since 2010 in Europe and Russia. When taking fingolimod, be wary of possible opportunistic infections and macular edema; The FDA recommends cardiovascular monitoring of patients, which is associated with an increased risk of bradyarrhythmia. A study design for fingolimod in pediatric MS patients was approved by the EMA in 2012 [56]. Currently, an international multicenter study of the use of fingolimod in children and adolescents with relapsing-remitting MS (PARADIGMS) is being conducted around the world and in Russia.
Teriflunomide was registered in Russia in 2014 for the treatment of MS from 18 years of age. The TERIKIDS trial is a two-year, multicenter, randomized, double-blind, placebo-controlled, parallel-group study designed to evaluate the efficacy, safety, tolerability, and pharmacokinetics of once-daily oral teriflunomide in children with relapsing forms of MS with an open-label follow-up period.
Cyclophosphamide, like rituximab, is not officially approved for use in MS in either children or adults. However, quite a lot of experience has been accumulated in the use of both drugs, including in pediatric patients [46, 61—67]. The safety profile is not entirely favorable, allowing for the possibility of infectious complications, menstrual irregularities, infertility and secondary malignancies for cyclophosphamide and cases of PML for rituximab. These data indicate the need to develop additional research to systematize existing experience and more accurately determine the place of these drugs in the treatment of MS.
Immunoglobulin G for intravenous administration (IVIG) also stands somewhat apart from other drugs used for MS in patients of different ages. But the results of using IVIG in neurological practice are very contradictory: a number of researchers observed a positive clinical effect, but without an effect according to MRI, others noted positive dynamics on MRI without changes in the clinical course of the disease. This may be due in part to the lack of clarity about the mechanisms of action of IVIG, as well as the different doses and characteristics of individual IVIGs. In general, in MS, IVIG is now considered the second choice for pathogenetic treatment after interferon-β and glatiramer acetate [69–71].
Conclusion
There are many unresolved issues in the study of MS in children and adolescents. Moreover, there are age-related features of the immune response [48], which can determine the pharmacodynamics and pharmacokinetics of different drugs. Therefore, the problem of the dependence of the effectiveness and safety of drugs for the treatment of MS on the age of patients remains relevant [51].
The IPMSSG believes that post-marketing research of promising drugs for the treatment of MS should be carried out with the aim of finding new effective and relatively safe treatments not only in adult patients, but also in children and adolescents. The design of pediatric studies should take into account all available information about the mechanism of action of the drug, data on its effectiveness and safety (see Appendix). Only a thorough analysis of systematic data will be able to answer questions about the effectiveness and safety of the use of DMTs in children and adolescents with R.S. Obtaining such data today is only possible through long-term multicenter randomized clinical trials. Therefore, all children and adolescents with definite MS [11] should be considered as potential participants in this type of research.
Application
IPMSSG Guidelines for Post-Marketing Study of Experimental Drugs for the Treatment of Pediatric MS [68]
1. The use of new therapeutic techniques in pediatric patients with MS is only possible in the context of carefully planned clinical trials. The widespread use of such drugs in the “off-label” mode hinders the conduct of modern, well-controlled therapeutic studies in pediatric patients.
2. New and experimental drugs with high potential efficacy and toxicity can be submitted for pediatric research only after obtaining reliable data on delayed safety based on use in adult patients. Re-examination of the spectrum of efficacy and safety in children and adolescents for such aggressive drugs is imperative.
3. New and experimental drugs with proven efficacy and safety after Phase III studies in adult patients should be re-examined in a pediatric group of patients.
4. Pediatric therapeutic studies may be conducted in tandem with phase III studies in adults, provided that data are available on a favorable efficacy-safety profile, positive experience in children and adolescents with a drug with a similar mechanism of action, or data on the use of this drug in pediatrics for the treatment of other diseases .
5. Placebo-controlled studies of pediatric MS should be of short duration and have clear and frequent monitoring of the neurological status of patients in order to early detect significant deterioration in the condition of patients receiving placebo in order to remove them from the study.
6. In situations where the need for specific therapy is limited to very small groups of patients (for example, in patients with malignant or resistant forms of MS), conducting a clinical trial may not be appropriate. In such cases, data on the use of the drug in each patient is combined into a common database, on the basis of which conclusions are drawn about its effectiveness and safety.
7. All pediatric studies conducted in patients with MS should be combined into a common data bank to collect and analyze information on the delayed effects of various treatment methods on the growth and development of patients.
For more information about IPMSSG members and policies, please visit www.ipmssg.org.
In Russia, the use of interferon-β-1a for subcutaneous administration is annotated from 12 years of age, interferon-β-1b is recommended for use up to 18 years of age with caution.
On the territory of the Russian Federation, on a legal basis, only drugs that have directly undergone clinical testing in patients under 18 years of age, and not their analogues, can be used in pediatrics.
Symptoms of Multiple Sclerosis
The clinical picture of this disease in children is very variable and depends on the location of the focus of demyelination and the course of the disease. Early signs of pathology are nonspecific and are often missed by parents. The main symptoms of multiple sclerosis in children include:
- A constant feeling of general fatigue , which does not depend on physical activity and daily routine. Children become inactive and stop playing with toys and other children.
- Visual impairment . Children often complain of the appearance of a “mesh before the eyes,” double vision, decreased visual acuity, and pain when moving the eyes. Sometimes visual fields are lost and color perception is impaired. Such episodes last more than a day with subsequent restoration of vision. With dystrophic changes in the optic nerve, irreversible damage occurs. When the oculomotor cranial nerves are damaged, strabismus and nystagmus occur.
- Local muscle cramps . They appear suddenly and disappear just as quickly.
- Hearing loss occurs when the auditory nerve or central auditory analyzer in the temporal lobes of the brain is damaged.
- Movement disorders such as central paralysis (increased muscle tone, decreased strength in the limbs, hyperreflexia, the appearance of pathological reflexes). Abdominal reflexes disappear in multiple sclerosis. Any type of paresis is possible: paraparesis, hemiparesis, monoparesis, tetraparesis. A characteristic feature of motor deficits in multiple sclerosis is variability. Weakness in the limbs waxes and wanes throughout the day.
- Cerebellar symptoms : impaired balance and coordination of movements, intention tremor, scanned speech, etc.
- Disorders of the sensitive area . The most common manifestation of this syndrome is a sensation of “crawling on the skin” or “tingling”. This symptom is called paresthesia. First of all, deep sensitivity suffers (muscular-articular, vibration, kinesthetic). A characteristic sign of sensory impairment in multiple sclerosis is the occurrence of paresthesia in the spinal region when the head is tilted forward.
- Dysfunction of the bladder and lower intestines are the most common reasons why parents of patients consult a doctor. Acute urinary retention or incontinence, neurogenic constipation or fecal incontinence significantly reduce the child's quality of life. These symptoms are associated with a loss of “central” control over the detrusor and sphincters.
- Cognitive and psychological disorders . Children's performance at school worsens, they become inattentive and irritable, and sometimes unemotional and depressed.
For multiple sclerosis, it is impossible to identify separate “classic” symptoms. Each sign of the disease correlates with the location of the degenerative process in the nervous system. Therefore, recognizing the disease in the early stages is very difficult. Especially in children, who are not always able to objectively assess their feelings.
Diagnosis of multiple sclerosis
Diagnosis of the disease involves visualization of foci of demyelination of the central nervous system using instrumental research methods (MRI with the introduction of intravenous contrast), confirmation of the autoimmune activity of the body after specific immunological tests and determination of functional disorders. MRI is considered the “gold standard” in diagnosing multiple sclerosis. This method allows you to identify the slightest foci of demyelination. MRI with contrast gives a clear picture of the pathology, in which the “old” and “new” foci of demyelination differ from each other. Based on these data, one can judge the activity of the disease.
Immunological tests are carried out to assess the activity of the pathological process. The biological material for these tests is mainly cerebrospinal fluid or blood. Detection of lymphocytic pleocytosis and increased myelin levels are nonspecific signs of multiple sclerosis.
An extended blood immunogram allows you to identify those disorders of the immune system that have become critical in the development of demyelinating disease of the nervous system.
The evoked potential method is becoming increasingly important in the diagnosis of multiple sclerosis. It allows you to detect demyelination of neurons at the initial stage of the disease. After analyzing the nature of the passage of the electrical impulse from the peripheral receptor to the central analyzer, the degree of damage to the components of this circuit is determined.
To verify the diagnosis of multiple sclerosis, W. McDonald’s diagnostic criteria are used all over the world. They compare the number of symptomatic episodes (attacks) and the conclusion of magnetic resonance imaging (MRI).
All results of additional research methods must correspond to the clinical picture of the disease. Multiple sclerosis is characterized by transient episodes of nervous system dysfunction that last at least 24 hours. Symptomatic attacks should appear no more than once a month.
Hereditary factor
MS cannot be called a hereditary disease, but there is a theory according to which genes also play a role in the development of MS. To date, about 20 genes have been identified that determine the structural features of nervous tissue and may be important in the development of MS. This is confirmed by statistics: up to 20% of patients with multiple sclerosis have relatives with multiple sclerosis. However, even in the case of an existing predisposition, multiple sclerosis may not develop if other predisposing factors do not contribute to this.
Treatment methods for multiple sclerosis
Treatment of children with this disease is medicinal. There are two directions of therapy: pathogenetic and symptomatic.
The goal of pathogenetic treatment is to correct the dysfunction of the immune system and reduce the activity of autoantibodies against one’s own cells of the nervous system. For this purpose, anti-inflammatory drugs, immunosuppressants, and immunomodulators are used.
To reduce the severity of symptoms of multiple sclerosis, muscle relaxants, anticonvulsants, amino acids, B vitamins, antidepressants, neuroprotectors, psychostimulants and nootropics are used.
Physiotherapy is prescribed in the remission stage. The goal of non-drug treatment is to prevent complications of multiple sclerosis. Plasmapheresis, massage, and physical exercises are used for this.
Psychotherapy is an important component of the treatment process. This direction ensures the child’s social adaptation and prevents the development of depression and cognitive disorders.
Consequences of multiple sclerosis
The progression of multiple sclerosis can provoke a number of complications from other organs and systems. The most common consequences include:
- Infectious diseases. Due to dysfunction of the pelvic organs, children often experience infections of the urinary tract (cystitis, urethritis) and kidneys (pyelonephritis, chronic kidney disease). Multiple sclerosis often leads to breathing problems. Deterioration of pulmonary ventilation can provoke the occurrence of aspiration pneumonia.
- Movement disorders up to the development of plegia.
- Decreased vision, blindness.
- Hearing impairment, deafness.
- Depression and other mental disorders.
- Disability.
Smoking
It's no secret that smoking is a bad habit. But few people know that smoking is an established factor in the development of multiple sclerosis. More often the disease is diagnosed in smokers. In addition, the harm from tobacco smoke directly affects the progression of the disease. The reason is that smoking reduces the immune response of T and B lymphocytes, and, as a result, plays an important role in the development of inflammatory diseases. It is no secret that smoking is a bad habit. But few people know that smoking is an established factor in the development of multiple sclerosis. More often the disease is diagnosed in smokers. In addition, the harm from tobacco smoke directly affects the progression of the disease. The reason is that smoking reduces the immune response of T and B lymphocytes and, as a consequence, plays an important role in the development of inflammatory reactions [10, 11].