In May 2021, the US Food and Drug Administration (FDA) approved Aimovig (international nonproprietary name: erenumab), the first fully human antibody for the treatment of migraine. This medicine, developed jointly by the pharmaceutical companies Novartis and Amgen, has a fundamentally different mechanism of action than the drugs traditionally used to treat migraine. Will Aimovig be able to significantly reduce symptoms and improve the quality of life of migraine sufferers? Let's try to figure it out.
Modern medicines
A special project about modern medicines, the history of their creation, development methods and development trends.
The special project partner, Cytiva, was formed as a result of the sale of the GE Healthcare Biopharma division to Danaher Corporation. Cytiva is a global provider of technologies and services that advance and accelerate the development and manufacturing of therapeutics. The company has a rich heritage dating back hundreds of years. Cytiva's clients conduct life-saving activities ranging from basic biological research to the development of innovative vaccines, biologics and novel cell and gene therapies. The company's mission is to provide the tools and services they need to do things better, faster and safer, leading to better outcomes for patients.
The first two articles of our special project “Modern Medicines” were devoted to general issues of how the drug industry developed [1] and how scientists search for new drug targets [2]. This article opens a number of news materials about interesting modern medicines.
How big is the problem?
Migraine, a neurological disease characterized primarily by severe attacks of headache, has been known for more than 3000 years [3]. However, despite the well-studied symptoms and manifestations, the causes of migraine have not yet been fully established. It was previously believed that migraine is caused by changes in cerebral circulation, but many researchers now suggest that this is not the main cause [4].
Current understanding of the pathogenesis of migraine involves the involvement of certain neural pathways and neuropeptides, particularly the trigeminal nerve and CGRP, a calcitonin gene-related peptide. Research has shown that excited nerve cells can activate the trigeminal nerve, leading to the release of CGRP and other neuropeptides. CGRP greatly dilates blood vessels, causing local swelling, and this attracts cells involved in the process of inflammation. With inflammation of the vessels of the skull and dura mater, proinflammatory molecules stimulate pain endings in the meninges, which leads to migraine (Fig. 1) [5–8].
This excitation of nerve cells, which triggers migraine, is associated with heredity and physiology, but today its causes are far from precisely determined [3]. It is only known that this process can be provoked by a variety of factors: from banal stress, fatigue and insufficient sleep to current hormonal levels, depressive states and medication use [9].
Figure 1. Pathogenesis of migraine
What happens during a migraine attack?
At the same time, migraine manifests itself not only as a headache: the symptoms of this serious illness are much more diverse, and often more painful. Debilitating migraine attacks can be painful and unbearable and sometimes last from several hours to several days, accompanied by nausea, vomiting and increased sensitivity to light, sound and smell [3], [10]. Such attacks deprive millions of people around the world of the ability to think, live and work normally. It is believed that every seventh inhabitant of the planet suffers from migraine today, and in the ranking of causes limiting the ability to work, this disease took second place in the world (according to 2021 data) [11], [12].
It is not surprising that migraine was included by the World Health Organization in the list of diseases that most disrupt the social adaptation of patients [3].
Material and methods
An open, randomized, prospective, comparative study was conducted in 60 patients with episodic migraine with and without aura aged 18 to 65 years. The study included patients with migraine, the diagnosis of which was established in accordance with the ICHD-3 beta diagnostic criteria (2013). The frequency of headache attacks was no less than 2 and no more than 15 days in 1 month, which excluded the participation of patients with chronic migraine. The study did not include patients under 18 years of age or over 65 years of age, patients with drug-induced headache, taking migraine prophylaxis or other specific anti-migraine drugs. Patients with other neurological or psychiatric pathologies, as well as with a history of coronary heart disease or cerebrovascular disease were also not included.
Two groups were formed using simple randomization. Group 1 included 30 patients, whose average age was 31.0±6.7 years ( p
<0.01), predominantly women - 90% (
n
= 27).
6 (20%) patients had migraine with aura and 24 (80%) without aura, with a frequency of 2.2±1.0 attacks per month ( p
<0.01). Patients of group 1 took L.P. Migrepam in accordance with the instructions for medical use of the drug in a therapeutic dose of 2.5 mg.
Group 2 also included 30 patients, of which 24 (80%) were predominantly women. The mean age in the group was 31.5±7.4 years ( p
<0.01).
9 (30%) patients had episodic migraine with aura and 21 (70%) without aura with an average frequency of 2.2±1.3 attacks per month ( p
<0.01). In group 2, patients were prescribed L.P. as a drug to relieve headache attacks. Sumatriptan at a dose of 50 mg.
A comparison of the baseline characteristics of the study groups is presented in Table. 2.
Table 2. Comparative analysis of clinical and demographic indicators of the study groups Note. Data are presented in number (%) or mean (SD) format. VAS - visual analogue scale. * - p-value - statistical significance of differences compared to the average value in the study groups (p <0.05). Note. Data are presented in number (%) or mean (SD) format. VAS - visual analogue scale. * — p-value — statistical significance of differences in comparison with the average value in the study groups (p<0.05)
When examined in the study groups, the average age was comparable and fell in the third decade of life; in terms of gender distribution, females predominated in both groups. According to diagnostic criteria, migraine without aura predominated in both groups, with comparable frequency and intensity of headaches.
Participation in the study included three follow-up visits.
During the first (baseline) visit, inclusion criteria were determined, a clinical neurological examination was performed, vital signs (blood pressure, heart rate) were recorded, and concomitant medications or other therapy were assessed. The intensity of headache, photo- and phonophobia was determined using a 10-point VAS scale. The criteria for assessing headache included the following characteristics: frequency, severity of associated symptoms, number of attacks relieved by triptans. In addition, we studied indicators of the degree of influence of headache on the general condition and quality of life (HIT-6 index - Headache Impact Test), time of disability associated with headache (HALT index - The Headache-Attributed Lost Time), and the degree of maladaptation caused by migraine (MIDAS). Patient satisfaction with the therapy used for migraine attacks was assessed (MIGRAINE-ACT - The Migraine Assessment of Current Therapy). On the 2nd visit after 30 days, while taking L.P. Migrepam and L.P. Sumatriptan was subjected to a clinical neurological examination and assessment of headache characteristics (frequency, severity of attack and associated symptoms when relieved with the appropriate triptan). During the visit, questionnaires HIT-6, HALT, MIDAS, MIGRAINE-ACT were filled out. At the 3rd visit, after 60 days, the patients were finally examined and all the above-mentioned questionnaires were filled out. The effectiveness of therapy for L.P. Migrepam and L.P. Sumatriptan was assessed using specially designed symptom diaries completed by patients during three consecutive attacks.
Clinical characteristics of the impact of headache on patients in the study groups during the baseline visit are presented in Table. 2. The average value of the degree of influence of headache on the general condition and quality of life (HIT-6 index) was 59.9±5.9 points in group 1, 55.9±8.9 points in group 2, ( R
<0.05), which corresponds to a significant impact of migraine on daily activities.
The severity of pain and the degree of maladjustment on the MIDAS scale in group 1 exceeded the value in group 2 ( p
<0.01), however, both indicators indicated the presence of a pronounced limitation of daily activity in patients of both groups (≥21 points corresponds to grade IV severity ) [8].
The HALT index over the last 3 months before the start of treatment in the 1st group was 27.5±4.7 days, and in the 2nd group - 22.4±7.4 days ( p
<0.01), which indicates on the strong influence of headache on the functional state in the study groups and the need for drug correction of headaches. During the examination, satisfaction with the previously used therapy for migraine attacks according to the MIGRAINE-ACT questionnaire in group 1 was noted by 9 (30%) patients, which was comparable to the value in group 2 - 11 (38%).
Statistical analysis was carried out depending on the distribution of the sample population using parametric tests of Student, Pearson and Fisher's exact test using Excel 2021 and the statistical software package Statistica 12. To present the obtained data, methods of descriptive statistics were used (with the calculation of average values, standard deviation, standard errors of the mean). The results were regarded as significant at p
-value <0.05.
Migraine treatment today
Unfortunately, today there is no medicine that can get rid of migraines once and for all. The drugs used can mainly reduce the intensity and number of attacks or reduce symptoms and relieve pain. In this regard, therapy with such drugs is divided into two groups:
- Abortive (acute) therapy - to relieve or reduce symptoms of migraine that has already appeared.
- Preventative treatment – to reduce the intensity, duration or frequency of attacks.
In this article we talk about drug treatment and prevention, but leave behind the scenes a variety of non-pharmacological treatments for migraine: from relaxation techniques to cognitive behavioral psychotherapy and transcranial electrical stimulation. Meanwhile, these methods in some cases can be an effective alternative to medications in case of resistance or contraindications to drug therapy, and sometimes effectively complement it in the combined treatment of migraines [13].
Abortion therapy
In the treatment of mild to moderate migraines, various analgesics and NSAIDs (non-steroidal anti-inflammatory drugs) are prescribed, including the well-known paracetamol, acetylsalicylic acid and ibuprofen, sometimes in combination with caffeine, codeine or psychotropic substances in combination medications [14], [15]. ]. A significant drawback is the possible reverse effect of uncontrolled use of analgesics: worsening headaches, which in such conditions can become daily [8], [15].
When it comes to dealing with more severe and prolonged attacks of migraine pain, the “heavy artillery” comes into play, namely ergot alkaloids and triptans. Triptans were the first drugs created specifically for the treatment of migraine; They began to be developed back in 1972. Even then it was known that the ergot alkaloid ergotamine can reduce migraine attacks, which was associated with its vasoconstrictor effect [16]. This drug remained the only specific treatment for migraine until, in 1991, rational research finally led to the emergence of sumatriptan, the first drug of a new class that acts in a similar way to ergotamine, but is more effective and safe (Fig. 2) [16].
Unlike its famous derivative, LSD, ergotamine itself does not have hallucinogenic properties.
Figure 2. Triptans, medications used to relieve migraines, are selective serotonin receptor agonists. They bind to and activate specific subtypes of these receptors (5HT1B, 5HT1D and 5HT1F). This leads to inhibition of the release of neurotransmitters from the presynaptic membrane of the trigeminal nerve endings (shown in yellow), located in the vessels of the head. At the same time, the release of the already mentioned CGRP, a key neurotransmitter that causes migraines, is also inhibited. This “neural” mechanism of action is believed to block the main pathological link in the development of migraine. It is also known that taking triptans selectively constricts the vessels of the dura mater, reduces the permeability of the vascular wall and reduces edema. Either way, these medications are effective—they reduce inflammation and pain, as well as the intensity of accompanying migraine symptoms [17]. The ergot alkaloids ergotamine and dihydroergotamine act in a similar way, although they are less selective - they can bind to dopamine and adrenergic receptors, which is why their effectiveness and safety are lower.
[18]
However, despite their good efficacy and safety profile, triptans may theoretically be dangerous for patients with coronary artery disease due to their vasoconstrictor effects, which limits their widespread use as therapy for migraine [16].
Preventive therapy
Drug prevention is indicated when migraines are regular (more than 2-3 times a month), and it must be said that today such therapy is not entirely safe and effective: it is associated with significant side effects and the development of rapid tolerance to treatment (tachyphylaxis) [14]. In this case, several classes of drugs are used, originally developed for other indications, and so far with a largely unclear mechanism of action for migraine. These medications include: tricyclic antidepressants, antiepileptic drugs, beta blockers, calcium channel blockers, ACE inhibitors and some others [14], [19].
By the way, you can read about many of the listed drugs, their direct indications, history of appearance and mechanism of action in the first article of our series “Three generations of drugs” [1].
Clinical analysis of triptans for the relief of migraine attacks
Migraine is a common form of primary headache that significantly impairs the quality of life of young, able-bodied patients. The prevalence of migraine in Europe and the United States averages 14%; migraine is much more common in women [1]. Migraine is characterized by an intense, paroxysmal, often unilateral headache lasting from 4 to 72 hours, aggravated by physical activity and accompanied by various neurological, somatic, emotional-affective and autonomic manifestations. There are 2 forms of migraine: migraine without aura and migraine with aura [2]. In most patients, migraine is hereditary, but neurochemical and neurogenic factors are also discussed among the underlying mechanisms. It has also been shown that patients with migraine are characterized by increased excitability of neurons in the cerebral cortex. An important mechanism that triggers an attack and determines the development and maintenance of pain is the activation of the trigeminovascular system (TVS). The mechanism of migraine aura is associated with the spread of a wave of excitation of cortical neurons (spreading cortical depression) from the visual cortex to the somatosensory and frontotemporal region. Spreading cortical depression determines the nature and sequence of aura symptoms: visual, sensory, speech [3]. Most migraine attacks occur suddenly. But often an attack can be provoked by a number of endogenous and exogenous factors (migraine triggers). Many authors believe that a combination of several triggers is necessary to trigger a migraine attack. Currently, about 60 different factors that provoke migraine attacks have been described [4]. The main factors that provoke a migraine attack: – psychophysiological: excessive stress, fatigue, poor or excessive sleep; – hormonal and somatic: menstruation, use of oral contraceptives, high blood pressure; – emotional: anxiety, depression, severe stress; – dietary: irregular meals, alcohol, chocolate, cheese, citrus fruits, etc.; – exogenous: weather and climate change, bright light, noise, strong odors, smoking, hot bath. Diagnosis of migraine is clinical, i.e. it is based on a detailed clinical interview and examination of the patient. Information about the age of onset of headache, family history, characteristics of the attack, provoking and alleviating factors, and the absence of signs of a secondary nature of the headache are extremely important. When establishing a diagnosis, one should rely on the diagnostic criteria of the International Classification of Headaches ICHD-3 beta [2]. With a typical clinical picture of migraine, additional studies are not indicated, since they are not informative. Paraclinical studies should be carried out only if there is a suspicion of the symptomatic nature of cephalalgia.
Basic strategies for relieving a migraine attack
The main goals of migraine treatment are to alleviate migraine, prevent chronicity and improve the patient’s quality of life. To achieve the above goals, it is necessary to follow several approaches [5]: 1) relief of migraine attacks; 2) preventive treatment; 3) lifestyle recommendations and behavioral therapy. Preventive therapy for migraine is carried out to patients if there are appropriate indications (frequent attacks of headaches, a high degree of patient maladjustment, complete ineffectiveness of symptomatic remedies, etc.). In general, 10 to 30% of patients require preventive medications to reduce the frequency of attacks. Meanwhile, the strategy for stopping attacks should be applicable to every patient who seeks advice, since the severity of migraine cephalalgia, as a rule, requires drug relief, and the vast majority of patients use drugs for each migraine attack. The goals of relieving a migraine attack are to reduce the intensity, duration of pain and accompanying symptoms (nausea, vomiting, phono- and photophobia, etc.) and normalize the patient’s general condition. Choosing the optimal remedy for relieving a migraine attack among the existing variety of pharmacological agents is not easy for every patient.
Pharmacological agents used to relieve a migraine attack:
• Drugs with a nonspecific mechanism of action: – analgesics (paracetamol, codeine); – non-steroidal anti-inflammatory drugs; – combination drugs. • Specific antimigraine drugs: – selective 5-HT1B/1D receptor agonists (triptans); – non-selective agonists of 5-HT1 receptors (ergotamine, dihydroergotamine). When choosing a specific drug to relieve an attack, the individual characteristics of the patient should be taken into account, such as the intensity of the headache, the rate of its increase, the presence of associated symptoms, the degree of maladjustment, previous experience and patient preferences. From this point of view, a stratified approach is used, which involves taking into account the most important characteristic of a migraine attack - the level of maladjustment of the patient. This approach is based on ranking migraine attacks according to severity and degree of impairment of patient adaptation [6]. For patients with mild attacks and a good level of adaptation, simple analgesics and NSAIDs are prescribed, possibly in combination with drugs that improve their absorption. Patients with severe and moderate attacks require the use of triptans. Another important principle for relieving a migraine attack is the early administration of an anti-migraine drug, which provides a more complete analgesic effect and a lower likelihood of relapse. In addition, as the attack progresses, most patients develop gastroparesis with impaired passage of oral medications into the intestine and their poor absorption. Early use of antimigraine drugs is important from this point of view [7]. Triptans were created specifically for the treatment of migraine attacks. They have high affinity for 5-HT1D and 5-HT1B receptors, and they lack activity towards adrenergic, dopaminergic, muscarinic, histamine and serotonin receptor subtypes. Currently, 7 representatives of the triptan class are known, 5 of them are registered in Russia (Table 1) [8].
Pathophysiological mechanisms of action of triptans in migraine: – narrowing of excessively dilated cranial vessels; – inhibition of the release of anti-inflammatory and vasoactive peptides; – inhibition of pain transmission at the level of the brain stem. Despite the fact that all drugs belong to the same class, they have different pharmacokinetic parameters. These differences are of great clinical importance as determinants of triptan efficacy. In 1990, sumatriptan was introduced into clinical practice. He was the first representative of the triptan class. In clinical studies to this day, sumatriptan is considered the “gold standard” for the specific treatment of migraine attacks. Subsequently, drugs with higher bioavailability and a fairly long half-life came into practice, which made it possible to avoid frequent use of the drug and recurrence of headaches.
Comparative analysis of triptans for the relief of migraine attacks
In a study by J. Pascual et al. An analysis was made of the use of zolmitriptan at a dosage of 5 mg for the relief of migraine attacks. 82 patients were studied, who relieved an average of 7 migraine attacks. Patients took oral and intranasal zolmitriptan and subcutaneous sumatriptan. The authors concluded that about 62.5% of patients preferred zolmitriptan due to its good efficacy [9]. A similar study was conducted in 2015, the authors of which concluded that a standard dose of triptans reduced the intensity of headaches within 2 hours in 42–76% of patients. Among triptans, the subcutaneous form of sumatriptan and tablet forms of zolmitriptan and eletriptan showed the best effectiveness [10]. The effectiveness of zolmitriptan for the relief of acute migraine was shown in a study by LC Chen et al. The authors demonstrated that zolmitriptan 2.5 mg was as effective for acute migraine as eletriptan 2.5 mg and sumatriptan 50 mg. Also, zolmitriptan was more effective than naratriptan 2.5 mg [11]. Zolmitriptan is a selective 5HT1 receptor agonist. It has high affinity for 5HT1B/1D receptors and moderate affinity for 5HT1A receptors. By interacting with 5HT receptors of intracranial vessels (including arteriovenous anastomoses) and sensory nerves of the TVS, innervating pain-sensitive intracranial structures, zolmitriptan causes vasoconstriction associated with suppression of the release of CGRP, VIP and substance P, and alleviates the course of a migraine attack, reducing the intensity of pain no later than than 1 hour after administration, reducing the severity of nausea, vomiting, photo- and phonophobia. The interaction of zolmitriptan with receptors through a negative feedback mechanism helps to reduce the release of serotonin and reduce its content in the synaptic cleft. In addition, zolmi triptan acts on the centers of the brain stem responsible for the development of migraine, which explains the sustained repeated effect in the treatment of a series of migraine attacks in one patient. After oral administration, zolmitriptan is quickly and well absorbed from the digestive tract; food intake does not affect the absorption of the drug. Absolute bioavailability is about 40%. The maximum concentration in blood plasma is achieved 2 hours after administration. The average half-life of zolmitriptan and its active metabolite is about 3 hours. Plasma protein binding is low (approximately 25%). The drug is metabolized in the liver with the formation of an active N-desmethyl metabolite and two inactive ones - indoleacetic acid and N-oxide metabolite. The agonistic effect of the active metabolite on 5HT1 receptors is 2–6 times greater than the parent compound. Upon repeated administration, no accumulation is observed. More than 60% of the administered dose is excreted in the urine, mainly in the form of an indoleacetic metabolite, and about 30% is excreted unchanged in the feces. The renal clearance of zolmitriptan and its metabolites in patients with moderate and severe renal failure is reduced by 7–8 times. It is known that the pharmacokinetics of zolmitriptan does not change in elderly people [12]. In the study by S.M. Spencer et al. Zolmitriptan has been shown to be effective in the treatment of migraine associated with menstruation and migraine with aura, persistent and/or recurrent migraine. Zolmitriptan was also effective in relieving migraine symptoms such as nausea, photophobia, and phonophobia. For the relief of migraine headache, zolmitriptan 5 mg had similar efficacy to sumatriptan 100 mg for a single attack but was generally more effective than sumatriptan 25 and 50 mg for multiple attacks. Zolmitriptan is generally well tolerated by patients and causes fewer unwanted side effects. The most common adverse events during zolmitriptan therapy are asthenia, dry mouth, nausea, dizziness, drowsiness, paresthesia, and chest pain [13]. A large, double, randomized trial compared the efficacy and tolerability of zolmitriptan (2.5 or 5 mg) and sumatriptan (50 mg) for the relief of up to 6 moderate to severe migraine attacks. The study involved 1522 patients, of which 500 patients took zolmitriptan 2.5 mg, 514 patients took zolmitriptan 5 mg, 508 patients took 50 mg sumatriptan. Overall, the reduction in headache intensity within 2 hours in these groups was 62.9%, 65.7% and 66.6%, respectively. There were no statistically significant differences between 50 mg sumatriptan and 2.5 mg zolmitriptan (p = 0.12) or 5 mg (p = 0.80). The authors concluded that zolmitriptan (2.5 or 5 mg) was as effective as sumatriptan 50 mg [14]. However, a comparative study showed higher effectiveness in relieving a migraine attack within 2 hours with zolmitriptan at a dosage of 2.5 mg than with sumatriptan at a dosage of 25 and 50 mg, although 3.3% of patients did not have clinically significant indicators. In this study, the authors found no differences in the incidence of side effects between zolmitriptan and sumatriptan [15]. A large comparative study of 1445 patients with migraine assessed the effectiveness of zolmitriptan (at a dose of 2.5 mg and 5 mg) and sumatriptan (at a dose of 25 mg and 50 mg) [16]. Since most modern triptans are characterized by relief of 2 attacks out of 3, this study analyzed the number of patients with a permanent effect (in 3 consecutive attacks). This indicator was significantly higher in the zolmitriptan group (Fig. 1).
Triptans are contraindicated in patients with coronary heart disease (CHD), a history of stroke, uncontrolled arterial hypertension (AH), or taking certain medications [17]. The issue of the cardiac safety of triptans has been specifically studied in large-scale clinical studies. One of the largest prospective population-based studies was conducted between 1995 and 1999 in 20 US states to identify the serious cardiovascular consequences of migraine and establish their possible association with the use of modern antimigraine drugs. It involved 130 thousand migraine patients and approximately the same number of patients who did not suffer from migraine headaches. In these groups, during the entire observation period, the frequency of significant cardiovascular events was studied: myocardial infarction, stroke, cardiac arrhythmias, unstable angina, transient ischemic attacks (TIA). The frequency of these events was also analyzed in those taking (50,383 patients) and not taking (80,028 patients) selective 5-HT receptor agonists. In general, patients with migraine had a higher incidence of coronary artery disease, cerebrovascular diseases, hypertension and hyperlipidemia, as well as unstable angina and TIA compared with persons without migraine headaches. It was shown that in the group of migraine patients, among those who used and did not use triptans, there were no significant differences in the incidence of myocardial infarction, stroke, cardiac arrhythmias, unstable angina and TIA, and the incidence of deaths in patients using triptans was even lower. These results led the authors to conclude that the use of triptans in migraine patients is not associated with an increased risk of significant cardiovascular events [18]. Thus, the study of these selective properties of triptans is of great practical importance for substantiating the cardiac safety of modern representatives of this group of drugs. A comparison of the results of clinical and experimental studies does not allow us to associate the appearance of so-called triptan-associated cardiac complaints with objective cardiovascular disorders in patients with migraine. To explain them, other possible non-ischemic causes of these disorders should be explored. Their presence may be due to generalized vasospastic reactions, impaired motility of the esophagus, pulmonary circulation or energy metabolism of the skeletal muscles of the chest wall, as well as the development of central sensitization processes [2]. Cases of serious cardiovascular events in both clinical trials and clinical practice are extremely rare and their occurrence is not associated with the fact that patients are using triptans to relieve headache attacks. The safety profile of triptans has been well studied. Triptans have a low risk of serious cardiovascular side effects in patients without evidence of coronary disease, based on clinical trial data. This allows triptans to be recommended to patients at low risk of coronary disease without prior assessment of cardiac status. Thus, various drugs can be used to relieve a migraine attack. Choosing the optimal remedy for relieving a migraine attack among the existing variety of pharmacological agents is not easy for every patient. From this point of view, a stratified approach based on ranking migraine attacks by severity and degree of impairment of patient adaptation is optimal. Patients with severe and moderate attacks require the prescription of specific drugs (triptans), given their high effectiveness in clinical studies. Thus, based on data on the effectiveness of zolmi-triptan in comparative studies, we can recommend this drug as a priority for the symptomatic treatment of migraine. Also, for successful treatment, dynamic monitoring of patients is necessary. It is extremely important to remember that it is permissible to use no more than 10 doses per month to relieve attacks in order to avoid drug abuse and the development of drug-induced headaches.
Targeted therapy for migraine: a solution to the problem?
Since migraine is a chronic disease and the effectiveness and safety of the drugs used for prevention is poor, there is clearly an unmet need in this area; however, there are few new drugs specifically developed for this indication [14].
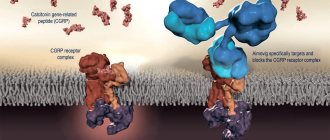
Amgen and Novartis can boast of a breakthrough today, having received approval in May 2021 for the use of the targeted drug Aimovig erenumab antibodies ) for the prevention of migraine in adult patients [20]. This is the first fully human antibody to be commercialized whose preventive effect is due to binding and blocking the CGRP receptor, which is considered critically important in the development of migraine (Fig. 3).
Figure 3. Aimovig specifically binds to the CGRP receptor, preventing CGRP from binding to it. The binding of CGRP to its receptor in a molecular complex (shown on the left) results in the transmission of pain signals associated with migraine. In people with migraine, CGRP levels are elevated during a painful attack and return to normal levels when the migraine pain subsides.
About Aimovig® (erenumab) in migraine prevention
Aimovig was approved after three series of clinical trials, the results of which, although slightly different, were similar in one thing: the drug showed a significant reduction in the number of days with attacks and the total duration of migraine pain. Moreover, in phase III trials, in 50% of patients taking 140 mg of Aimovig, the number of painful days decreased by half compared to placebo, and the safety and tolerability of the drug turned out to be quite similar to placebo [21], [22]. . These results are very encouraging, and it is clear that although Aimovig is not a panacea, its use will definitely change the lives of patients suffering from frequent and severe migraines for the better.
Antibody purification using continuous countercurrent chromatography
Biotechnologically derived antibodies can be purified using the chromatography equipment of this material's partner, Cytiva. This is done in continuous counter-current chromatography (PCC) mode on an ÄKTA pcc . In combination with the minimal dimensions of the system, continuous chromatography technology ensures the most efficient use of the chromatographic sorbent (resin). This method is particularly suitable for the purification of unstable molecules, as the rapid process ensures the stability of the target product. The ÄKTA pcc system is based on the well-established and widely used ÄKTA chromatography system platform by scientists around the world. Key features of the ÄKTA pcc system:
- Flow rate: 0.5–75 ml/min.
- Maximum operating pressure: 20 bar.
- Supports three and four column PCC configuration (3C and 4C PCC).
- Integrated UV detectors: one tunable wavelength (three values) and three fixed wavelength (280 nm).
- Monitors for tracking pH and conductivity readings.
- Interactive visualization that continuously shows column status and process progress.
- Trend lines that allow you to immediately begin analyzing the information received about the process.
ÄKTA chromatography systems can be used in conjunction with AxiChrom columns, although they are also suitable for larger scale protein purifications. A wide range of column sizes of the same type (Figure 4) allows protein purification to be scaled up with minimal parameter changes.
Figure 4. AxiChrom is a family of pilot-scale to industrial-scale protein purification columns with i.d. diameters of 50–1600 mm. AxiChrom columns successfully combine standardization and customization and are compatible with a variety of advanced media, such as MabSelect SuRe™ antibody purification resin.
Cytiva's research has confirmed the similarity between the protein purified in the laboratory and in production. In this case, the volume of the drug was scaled by 294 times (Fig. 5).
Figure 5. Comparison of chromatograms obtained using ÄKTA pcc on HiTrap columns (a) and AxiChrom 50 columns (b)
Material provided by our partner - Cytiva
results
Dynamics of headache intensity and accompanying symptoms in the study groups
Analysis of the main objective and subjective indicators characterizing the intensity of pain, accompanying symptoms and parameters of daily functioning showed that both triptans are equally effective as a symptomatic means of relieving both pain and accompanying symptoms of migraine.
Thus, the use of L.P. Migrepam at a dose of 2.5 mg was accompanied not only by a decrease in the intensity and duration of the headache in the first 2 hours, but also by a reduction in the severity of accompanying symptoms (photo- and phonophobia, nausea, vomiting) already in the first hour from the onset of relief of the attack, which led to a decrease the degree of maladaptation of patients, improving the quality of life and rapid return to social activity. It should be noted that the positive changes detected were recorded in the form of a decrease in the intensity of headaches after taking L.P. Migrepam during the first 30 minutes from 8.9 to 6.5 points according to VAS ( p
<0.001), and after 2 hours to 1.9 points on VAS (
p
<0.001), which corresponded to easily tolerated pain or pain that does not require analgesics. During the observation period of 24 hours, no recurrent migraine attack was observed (Fig. 1).
Rice. 1. Dynamics of headache intensity, photo- and phonophobia during a migraine attack (on the VAS scale). At the time of the full-blown attack, 85% of respondents indicated nausea, 40% indicated vomiting once or twice, and in 70% of cases the attacks were accompanied by photo- and phonophobia. In the first 30 minutes after taking L.P. With migrepam, a significant reduction in the severity of vomiting was noted (only 10% of patients indicated its presence) and its complete cessation was observed within 60 minutes after taking the drug. A 3-fold decrease in the incidence of nausea was noted in the first 60 minutes after taking the drug - only 29% of patients noted a persistent mild subjective feeling of nausea, and after 2 hours of taking L.P. With migrepam, the feeling of nausea persisted in 10% of patients ( p
<0.001), followed by complete relief within 2 hours (Fig. 2).
Rice.
2. Dynamics of the intensity of nausea and vomiting during a migraine attack (on the VAS scale). Complaints about photo- and phonophobia in patients decreased within 60 minutes by more than 50%; persistent accompanying symptoms were indicated by 21% of respondents, with complete regression of complaints within the second hour ( p
<0.001) (see Fig. 1).
In the 2nd group, in which L.P. was used. Sumatriptan at a dose of 50 mg, headache intensity decreased during the first 30 minutes from 8.5 to 4.7 points according to VAS ( p
<0.001).
By the end of the first hour, the headache intensity averaged 2.4 points, which corresponded to a mild headache; after 2 hours, patients indicated complete relief of the attack ( p
<0.001) (see Fig. 1).
The most common symptoms accompanying migraine pain in patients of group 2, comparable to group 1, were nausea (92%), photophobia (85%), phonophobia (77%), vomiting (46%). After taking L.P. Reliable results were also obtained with sumatriptan in the first 60 minutes: a decrease in the severity of vomiting (complete relief of the symptom within an hour). 33% of respondents indicated a persistent slight urge to feel nauseous. 2 hours after taking L.P. With sumatriptan, the feeling of nausea persisted in 8% of patients ( p
<0.001), followed by complete relief of the associated symptom within 2 hours (see Fig. 2).
Reliable indicators were obtained for such common symptoms as photo- and phonophobia. A decrease in photo- and phonobia occurred in patients during the first 60 minutes ( p
<0.001). During the 24-hour observation period for patients in group 2, there was no resumption of a repeated migraine attack (see Fig. 1). Based on the data obtained, a preliminary conclusion can be made: 2.5 mg L.P. Migrepam eases the course of a migraine attack, reducing the intensity of pain and accompanying symptoms no later than 2 hours after taking the drug, not being inferior in effectiveness to L.P. Sumatriptan.
Dynamics of HIT-6 index parameters in the study groups
During the 1st (baseline) visit, the average value of the degree of influence of headache on general condition and quality of life (HIT-6 index) in both groups was comparable and corresponded to the significant impact of migraine on daily activities (see Table 2). After 30 days of treatment (2nd visit), in group 1 there was a decrease in the average value on the HIT-6 index to 51.7±5.0 points ( p
<0.001) and with a further decrease in the score by the 60th day to 47.7±3.6 points (
p
<0.001), which corresponded to a low level of influence of headache on everyday life.
In group 2, at the 2nd visit, the HIT-6 index decreased from 55.9±8.9 to 52.2±7.8 points ( p
<0.001), and by the 3rd visit this indicator was 45.6 ±5.6 points (
p
<0.001).
The differences in the study groups were not significantly different at the 3rd visit ( p
< 0.05) (see Fig. 3).
Rice. 3. Dynamics of HIT-6 index parameters in the study groups.
Dynamics of the MIDAS scale in the study groups
In both study groups, by the 3rd visit during treatment, a significant moderate decrease in the degree of influence of recurrent migraine attacks on daily life activities was observed. By the 2nd visit in group 1, the value on the MIDAS scale was 26.8±8.2 points ( p
<0.005), and at the 3rd visit - 22.6±9.1 points (
p
<0.001).
The dynamics on the MIDAS scale in group 1 were not as significant as expected, in contrast to group 2, where there was a statistically significant decrease in the score to 16.9±11.7 points ( p
<0.001) (Fig. 4) .
Rice. 4. Dynamics of MIDAS scale parameters in the study groups.
Dynamics of HALT index indicators in the study groups
In addition to improving indicators that directly characterize the degree of influence of headache on the general condition and quality of life of patients, with the use of L.P. Migrepam was associated with positive changes in other measures, including the HALT index, which measures time lost due to headaches, and the need to re-evaluate the medication used. During treatment in group 1, there was a reduction in the average value on the HALT scale by the 2nd visit to 25.4±8.2 days ( p
<0.05) and by the 3rd visit up to 20.2±6.6 days (
p
<0.01).
In group 2, at the 2nd and 3rd visits, there was also a decrease in the HALT index from 22.4 ± 7.4 to 14.3 ± 4.9 days in comparison with the initial indicator ( p
<0.01).
The differences in the study groups by the 3rd visit were significantly different in the 2nd group ( p
<0.01), however, both indicators corresponded to the value of average or moderate limitation of daily activities due to headache (Fig. 5).
Rice. 5. Dynamics of HALT index parameters in the study groups.
Dynamics according to the MIGRAINE-ACT questionnaire in the study groups
In the present study, the use of L.P. Migrepam caused the greatest adherence and satisfaction with the quality of treatment among patients. When examining patients, their satisfaction with previously used therapy for migraine attacks according to the MIGRAINE-ACT questionnaire in both study groups at the time of the initial (1st) intake did not reveal significant differences. However, after 30 days while taking L.P. With migrepam, the number of patients satisfied with treatment was 50% ( p
<0.001).
By the 3rd visit, there was a significant increase in the relative number of patients assessing the quality of their treatment as “satisfactory”, up to 70% of respondents ( n
= 21), which exceeded the value in the comparative analysis in the 2nd group, in which attacks were relieved by taking L .P.
Sumatriptan, the relative number of patients assessing their treatment as “satisfactory” at the 2nd visit was 46% ( n
= 14), and at the 3rd visit - 62% (
p
< 0.001) (Fig. 6).
Rice. 6. Dynamics of subjective assessment of the relief of migraine attacks according to the MIGRAINE-ACT questionnaire in the study groups.
Dynamics of relief of 3 subsequent attacks in the study groups
During therapy, the number of patients with complete relief of three consecutive migraine attacks was assessed. In group 1, among patients who used L.P. Migrepam, relief of 2 out of 3 attacks was noted by 31%. Moreover, 53% of the patients indicated relief of all 3 attacks. In group 2, against the background of the use of L.P. With sumatriptan, the number of patients who noted relief of 2 attacks out of 3 was 33%, which did not differ significantly from this indicator in group 1. To stop 3 consecutive attacks while taking L.P. Sumatriptan was indicated by 47% of patients, which was significantly lower than this indicator in group 1 ( p
<0.05). The percentage of patients resistant to both triptans was comparable in both groups and amounted to 8 and 7, respectively (Table 3).
Table 3. Analysis of relief of 3 consecutive migraine attacks in the study groups.
Subjectively, good tolerability of drug therapy with both triptans was noted. The frequency of adverse reactions (nausea, dry mouth and paresthesia in the extremities, sensations of warmth and heat) did not differ significantly between the groups and amounted to 11.9% in group 1 and 12.1% in group 2. All side effects were mild, went away on their own and did not require additional therapy.
"Aimovig" and competitors
It must be said that Novartis and Amgen already have serious competitors in the field of targeted anti-migraine therapy: Eli Lilly released Emgality, and Teva Pharmaceutical released Ajovy. These drugs are humanized monoclonal antibodies that target the ligand rather than the CGRP receptor, and in this way they differ from Aimovig, being inferior to the latter at least in tolerability. When using Ajovy, 43–45% of patients experienced pain, redness and itching at the injection site, when using Emgality, 18% of patients experienced such symptoms, and Aimovig caused such reactions only in 5–6% of cases. At the same time, Aimovig is also more convenient to use, since of the three listed drugs, only it comes in the form of an auto-injector - a device that resembles an insulin syringe pen - which needs to be used only once a month. The listed advantages, coupled with the fact that Aimovig became the first targeted drug for the treatment of migraine and immediately filled an empty niche in the market, will most likely provide it with a bright financial future: according to experts, its market share in 2024 will be $2 billion, while the predicted shares of competitors are approximately half as much [20].
The success of Aimovig, apparently, cannot be hampered even by the extremely high price ($575 per month according to the Amgen price list - however, in the United States, the drug is covered by insurance for many patients), which, by the way, some experts consider unreasonable [11]. The company is conducting research on Aimovig in Russia, but when the drug will appear on the market, how much it will cost and who will pay for it is still unknown.
Literature
- Three generations of drugs;
- Search for drug targets;
- Sanoeva M.Zh. and Saidvaliev F.S. (2016). Migraine - yesterday, today, tomorrow. A modern view of the problem. "International Neurological Journal". 8, 72–78;
- How a migraine happens. Johns Hopkins Medicine;
- Migraine headaches. (2018). WebMD;
- Paul L. Durham. (2006). Calcitonin Gene-Related Peptide (CGRP) and Migraine. Headache
.
46 , S3-S8; - What to know about the new anti CGRP migraine treatment options. (2018). American Migraine Foundation;
- Silberstein S. D. (2016). Migraine. MSD Directory;
- Migraine triggers: your personal checklist. (2019). WebMD;
- Rusakova E. (2018). The FDA has approved the drug to prevent migraines. N+1;
- Kolata G. (2018). The FDA approves the first drug designed to prevent migraines. The New York Times;
- Theo Vos, Amanuel Alemu Abajobir, Kalkidan Hassen Abate, Cristiana Abbafati, Kaja M Abbas, et. al.. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet
.
390 , 1211-1259; - Francesca Puledda, Kevin Shields. (2018). Non-Pharmacological Approaches for Migraine. Neurotherapeutics
.
15 , 336-345; - Doodipala Samba Reddy. (2013). The pathophysiological and pharmacological basis of current drug treatment of migraine headache. Expert Review of Clinical Pharmacology
.
6 , 271-288; - Ogbru A. and Ogbru O. Prescription migraine medications. RxList;
- Boyce S., Ali Z., Hill R. G. (2001). New developments in analgesia. Drug Discovery World;
- Azimova Yu.E. (2017). Triptans: an era of specific treatment for migraine. "Nervous diseases". 1, 10–14;
- Ameri M. and Lewis H. (2018). The evolution of transdermal drug delivery and treating migraine. Drug Discovery World;
- Migraine medications. (2013). TMedWeb;
- Dmitriev R. (2019). "Aimovig" will turn chronic migraine into episodic. "Mosmedpreparaty";
- Aimovig (erenumab) for the treatment of episodic migraine. Clinical Trials Arena;
- Khoruzhaya A. (2018). A new anti-migraine drug goes on sale. "Neuronews";
- Targeted therapy is a targeted strike against the disease.