Article for the “bio/mol/text” competition: Epilepsy is one of the widespread nervous diseases, characterized primarily by the occurrence of seizures. Her therapy includes long-term (often lifelong) use of antiepileptic drugs (AEDs). Up to 40% of those suffering from epilepsy are women of childbearing age. It is especially dangerous for epileptic mothers that they have to take high doses of PeP during pregnancy. Taking PEP medications (for example, valproate) can lead to disturbances in the development of the embryo - the so-called. fetal valproate syndrome, which leads to structural defects in almost all body systems, developmental delays and mental disability.
Epilepsy and its therapy
Figure 1. Henry Perche: Healing a Woman with Epilepsy in a Sequential Series of Images , from the Book of the Deeds of St. Louis, 15th century.
website epilepsiemuseum.de
Epilepsy is a pathology known to mankind in ancient times (falling sickness is one of the first names for this disease; it was treated by expelling demons from the victim’s body - they say it helped some; Fig. 1). It can affect sensory, motor, autonomic functions, memory, behavior, emotional status, perception, speech, behavior. The very definition of the disease caused controversy for a long time, until finally, in 2005, the International League Against Epilepsy approved the following postulates:
- Epilepsy is a general name for a whole group of diseases that reflect the consequences of brain dysfunction.
- Epilepsy is a brain disorder characterized by a persistent predisposition to seizures and their neurobiological, cognitive, psychological and social consequences.
- An epileptic seizure is a transient manifestation of signs and/or symptoms related to abnormally excessive or synchronous neuronal activity in the brain.
- At least one epileptic seizure is required to make a diagnosis.
Today, about 2% of people worldwide suffer from epilepsy, making it one of the most common neurological diseases. Without going into details, the occurrence of an epileptic seizure is caused by an imbalance of the inhibitory (the main neurotransmitter is γ-aminobutyric acid, GABA) and excitatory (the main neurotransmitter is glutamate) systems of the brain [8]. Before a seizure, the action of glutamate greatly overpowers the action of GABA in any area of the brain, and the neurons of this area begin to generate electrical impulses not separately, as is usually the case, but synchronously. The so-called epileptic focus, where cells discharge simultaneously. An electroencephalogram (EEG) shows a terrifying picture (Fig. 2).
Figure 2. Comparison of electroencephalograms of a healthy patient and a patient with juvenile myoclonic seizures (one of the forms of hereditary epilepsy that manifests itself in puberty).
Children's hospital of Pittsburgh
Taking medication is currently the first and most important part of treating epilepsy. It is usually recommended to start with monotherapy with antiepileptic drugs (AEDs), which can suppress seizures or at least minimize the frequency of seizures in 50–70% of patients. To treat patients who do not benefit from monotherapy, polytherapy is used, i.e. a combination of several anticonvulsants. Commonly used AEDs include valproic acid, vigobatrin, tiagabine, lamotrigine, oxcarbamozepine, felbamate, topiramate, gabapentin, levetiracetam, zonisamide, and pregabalin. If drug treatment does not help in this case, then extreme measures are used: surgical excision of the epileptogenic focus, a ketogenic diet and deep vagal stimulation of the brain [16], [19].
For many AEDs, their anticonvulsant (i.e., preventing the onset of a seizure) effect was first shown, and then the specific mechanism by which the drug prevents the development of a seizure began to be elucidated. For example, a drug can interfere with voltage-sensitive ion channels and prevent the cell from generating an action potential; can enhance the influence of the GABAergic system, cause a weakening of the influence of the glutamatergic system through mediator receptors, and can directly affect the release of mediators. Some drugs are characterized by a combination of several of the above mechanisms [8], [16].
However, simultaneously with the study of the therapeutic activity of the drugs, serious side effects characteristic of PeP began to emerge. Therefore, treatment in each case must be selected individually, taking into account the characteristics of the individual patient. Epileptic women of childbearing age represent a special risk group, because for them the question is not only how to get pregnant (PePs change the body’s metabolism and its hormonal levels), but also how to maintain the health of the unborn child - after all, all PePs are teratogenic, i.e. cause both physical and mental defects in the child.
Valproic acid
One of the most widely used and studied drugs for the treatment of epilepsy is valproic acid. It helps patients in different doses with almost all types of seizures, at any age, and it is what doctors prescribe when they need to stop (block) a seizure in a patient with an unknown etiology of the disease.
Figure 3. Valproic acid. On the left is the chemical structure of valproic acid. On the right is Valerian officinalis, from which it is obtained.
Valproic acid (found in the literature as VPA or valproate salt anion) is a derivative of valeric acid obtained from the essential oil of the herbaceous plant Valeriana officinalis. VPA was discovered in 1882 by B. Burton, but it was not until 1963 that its anticonvulsant effect was discovered. Today, valproic acid is used not only in the spectrum of epileptic diseases, but also in the treatment of bipolar disorders, senile dementia, leukemia, schizophrenia, migraines, and depression. The antiepileptic effect of the drug is usually associated with the fact that it increases the inhibitory activity of GABA through an increase in its synthesis and a decrease in degradation. Valproate has also been shown to have a neuroprotective effect [1], [8]. It is known that in the area of the seizure focus, neurons are damaged, but when VPA is administered before a seizure or immediately after it, the proportion of dead cells is significantly reduced, and the supply of nutrients to cells (neurotrophism) is enhanced. (In particular, this effect was observed in the hippocampus, the part of the brain responsible for the formation of memory and spatial orientation.)
However, treatment with valproate also has a direct negative effect on the body of an epileptic patient, and the higher the dose of the drug, the more disorders are detected in the body. If we talk about the most common manifestations, these are weight gain, metabolic complications, decreased nighttime sleep duration, pancreatitis, acute liver failure, coagulopathies, various hypersensitivity reactions, encephalopathy, emotional disorders, depression, dysfunction of the endocrine and reproductive systems. The latter is especially pronounced in women: polycystic ovary syndrome, disturbances in the pharmacokinetics of oral contraceptives, and menstrual irregularities [2], [19]. In addition, VPA complicates the process of embryo implantation in the initial stages of pregnancy, reducing the expression of proteins necessary for this (laminin, collagen-IV and vimentin) [6]. And it is valproic acid that is the most dangerous drug for pregnant women: it easily penetrates the placental barrier [3] and causes many irreversible changes in the fetus.
In 1984, fetal valproate syndrome was first described [4], which is characterized by the occurrence of malformations (structural defects; Fig. 4) of the cardiovascular and genitourinary systems, skeleton, and facial features; abnormalities appear in the eyes and central nervous system (the most typical for valproate is non-closure of the neural tube), and the respiratory tract [7], [18]. Physical development itself slows down after birth: children weigh less and grow more slowly than their peers. The frequency of such events with VPA monotherapy is three times higher compared to monotherapy with any other AED.
Figure 4. Malformations in fetal valproate syndrome.
[7]
The question arises: what can cause more harm to a child? An epileptic seizure of the mother during pregnancy (and this means, at a minimum, a sharp decrease in the supply of oxygen to the fetus’s brain, and at a maximum, its death) or drug therapy to prevent the development of this seizure? Looking at the statistics calculated for people, this question is almost impossible to answer. Therefore, studies are carried out on animal models, in particular rats.
The author of this article took four groups of rats:
- Control group.
- Group receiving intraperitoneal sodium valproate throughout the first trimester of pregnancy.
- A group in which epileptic seizures were induced twice during the first trimester - before and after implantation of the embryo into the wall of the uterus - by the classic chemical convulsant pentylenetetrazole (PTZ), an inhibitor of postsynaptic inhibition of neurons in the central nervous system.
- The group that received both sodium valproate every day of the first trimester and PTZ was administered twice (but did not develop seizures against the background of VPA).
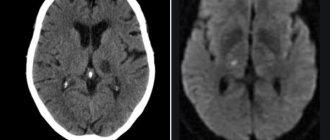
It turned out that adverse pregnancy outcomes were present in all groups except the control group (for the group receiving VPA - 87.8%; for the group exposed to epileptic seizures due to the administration of PTZ - 50%, for the group receiving both VPA and PTZ, - 100%), however, only in rat pups exposed only to VPA in utero, a strong slowdown in physical development was observed (criteria: eye opening, weight gain and growth up to adulthood) and an increase in anxiety in behavioral tests. Also, MRI diagnostics was carried out on the rat pups when they were already grown up, which again revealed disorders mainly in the group that received only valproate: in Fig. 5 large white spots on the top line of the drawings mean expansion of the ventricles of the brain. The volumes decrease and the cellular structure of many parts of the brain changes:
- thalamus (mediator of the transmission of sensory and motor signals to the cerebral cortex from the lower parts of the nervous system);
- globus pallidus and striatum (these are the basal ganglia, part of the extrapyramidal system, are involved in the planning and execution of movements, and influence muscle tone);
- amygdala (part of the limbic system, responsible not only for the formation of positive emotions, but also for feelings of fear, aggression, anxiety);
- hippocampus (responsible for spatial orientation and rewriting information from short-term memory to long-term memory, its volume significantly decreased in all groups compared to the control).
Figure 5. Tomographic sections of the brain of two-month-old rats receiving VPA prenatally in the first trimester (A-D) and rats from the control group (E-H), T2 RARE mode. Expansion of the ventricles of the brain in the experimental group is clearly visible.
research by the author of the article
Thus, we can say that the prenatal effect of valproate can affect the physical development of the fetus and change the structure of the brain for the rest of life, affecting motor functions, the process of formation of emotions, memory, and behavior in general, and this effect much more destructive than the effect of the seizure itself.
Other studies found that rat pups exposed in utero to VPA (dose 600–800 mg/kg) weighed less, their development slowed down, they had decreased pain sensitivity and, on the contrary, increased sensitivity to non-painful stimuli, locomotor hyperactivity and stereotypy in combination with low research motivation, reduced level of social behavior; they showed reduced acoustic prepulse inhibition (i.e., decreased motor response to a sharp sound). Typically, the same disorders are detected in autism pathologies [12], [14].
Nowadays, the effects of valproate are being increasingly studied not only in animal models, but also in humans, studying statistics of deviations and conducting psychological tests. The correlation between the intrauterine influence of HPA and the development of autism spectrum diseases can be considered proven: IQ in children of preschool and school age and the tendency to communicate are below normal, attention and learning are impaired [11], [15], [17].
Medicines for the treatment of COVID-19
The Ministry of Health of the Russian Federation periodically updates guidelines for the prevention, diagnosis and treatment of a new type of coronavirus. Recently, the list of possible drugs for use in treating the disease in adults has been expanded. Analysis of the literature data on clinical experience in the management of patients with atypical pneumonia associated with the SARS-CoV and MERS-CoV coronaviruses allows us to identify several etiotropic drugs that are recommended for use in combination. These include chloroquine, hydroxychloroquine, lopinavir + ritonavir, azithromycin (in combination with hydroxychloroquine), interferon drugs. Among the drugs that are at the stage of clinical trials in patients with COVID-19, umifenovir, remdesivir, and favipiravir can also be noted [4].
In world practice, the following antiviral and antiparasitic drugs are used in the treatment of COVID-19.
Atazanavir
(Atazanavir) is an antiviral agent that is an azapeptide inhibitor of HIV protease. Selectively inhibits virus-specific processing of viral Gag-Pol polypeptides in HIV-infected cells, preventing the formation of mature virions and infection of other cells. Safe for use during pregnancy [5]. Along with other protease inhibitors, HIV is used as an experimental therapy for SARS-CoV-2 [6].
Lopinavir/ritonavir
(Lopinavir/ritonavir) is a combination antiviral drug. Lopinavir is an HIV-1 and HIV-2 protease inhibitor and provides antiviral activity to this combination. Inhibition of HIV proteases prevents the synthesis of viral proteins and prevents the cleavage of the Gag-Pol polypeptide, which leads to the formation of an immature virus that is incapable of infection. Ritonavir inhibits the metabolism of lopinavir in the liver mediated by the CYP3A4 isoenzyme (cytochrome P450 3A4), which leads to an increase in the concentration of lopinavir in the blood plasma; ritonavir is also an inhibitor of HIV protease. The combination of two drugs neutralized the side effects of individual drugs [7]. This combination antiviral drug is used to treat COVID-19, but has shown low effectiveness in severe forms of the disease [8].
Darunavir/cobicistat
(Darunavir/cobicistat). Darunavir, like lopinavir, is an HIV protease inhibitor, and cobicistat increases the effectiveness of darunavir by blocking the CYP3A isoenzyme. Used as an experimental drug for the treatment of COVID-19. However, studies have shown its ineffectiveness in preventing COVID-19 in patients with HIV, and the effectiveness of this drug against SARS-CoV-2 has also been questioned [9].
Remdesivir
(Remdesivir, development code GS-5734) is a new antiviral agent developed jointly by the US Army Medical Research Institute of Infectious Diseases and the American company Gilead Sciences for the treatment of Ebola and Marburg viruses. Remdesivir reduces the production of viral RNA, and in the case of the Ebola virus, it breaks the chains of viral RNA and is capable of causing mutations that inactivate the virus. Studies have shown that the substance also affects other single-stranded RNA viruses, such as respiratory syncytial virus, Junin virus, Lassa fever virus, Nipah virus, Hendra virus and coronaviruses (including MERS and SARS viruses) [10].
Favipiravir
(Favipiravir) is an antiviral drug, a selective inhibitor of viral RNA-dependent RNA polymerase, also induces lethal RNA transversion mutations, producing a nonviable viral phenotype [11]. Since 2014, it has been approved for use as an anti-influenza drug in Japan. It is used as an experimental drug for the treatment of COVID-19. It is prohibited for use in pregnant women, since preclinical studies revealed teratogenic and embryotoxic effects [12].
Chloroquine
(Chloroquine) is an antimalarial drug from the group of 4-aminoquinoline derivatives.
It inhibits the synthesis of nucleic acids in cells and has a moderate immunosuppressive, specific and nonspecific anti-inflammatory effect. It is used for the prevention and treatment of malaria, causing the death of epithrocytic forms of most types of plasmodia (except Plasmodium falciparum
), and is also effective against
Entamoeba histolytica
. As an immunosuppressive and anti-inflammatory drug, it is effective in rheumatoid arthritis, systemic lupus erythematosus, autoimmune glomerulonephritis, sarcoidosis, scleroderma, and photodermatoses [13]. It is used as an experimental drug in the treatment of COVID-19 [14].
Hydroxychloroquine
(Hydroxychloroquine) is an antimalarial drug, a derivative of 4-aminoquinoline. It also has a moderate immunosuppressive, specific and nonspecific anti-inflammatory effect in autoimmune diseases, which expands its indications for the treatment of many systemic diseases. The drug is used in the treatment of COVID-19, especially complicated by the presence of diabetes mellitus [15]. It is most effective in combination with azithromycin, promoting accelerated elimination of the virus from the body, as well as in the fight against attached bacterial flora in the prevention of pneumonia [16]. Hydroxychloroquine is included in the list of vital and essential drugs and was approved by the Russian Ministry of Health for the treatment of coronavirus infection [4].
Nitazoxanide
(Nitazoxanide) is an antiprotozoal and antiviral drug, most often used against hepatitis B and C viruses. There is evidence that the drug has an anticancer effect (for oncological processes in the prostate, ovaries and intestines) [17]. Used as an experimental drug for COVID-19 [18].
Ribavirin
(Ribavirin) is an antiviral drug active against a number of DNA and RNA viruses, is an antimetabolite of nucleosides and prevents the replication of viral genomes. Ribavirin has been shown to be effective against influenza viruses and many viral hemorrhagic fevers; it is used to treat severe infections caused by respiratory syncytial virus, viral hepatitis C, etc. Ribavirin is active in the form of a metabolite, which has a structure similar to the purine nucleotide guanine. One of the side effects of ribavirin is hemolytic anemia, which can be fatal (this side effect is dose-related). Ribavirin has also been shown in preclinical studies to have a teratogenic effect, so it is prohibited for use in pregnant women [19]. Ribavirin is used as an experimental drug for COVID-19 [18].
Tocilizumab
(Tocilizumab) is an immunosuppressant used primarily to treat rheumatoid arthritis and systemic juvenile idiopathic arthritis. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R), binds soluble and membrane IL-6 receptors, preventing the pro-inflammatory effect, and blocks the occurrence of the so-called “cytokine storm”, which causes shock and hypoxemia. The drug was successfully tested by Chinese specialists against COVID-19 [20].
Oseltamivir
(Oseltamivir) - oseltamivir phosphate, which is a “prodrug” that is converted in the body to oseltamivir carboxylate, which is an inhibitor of neuraminidase, used by influenza virions types A and B to exit the cell. Oseltamivir is also used as an experimental drug for the treatment of COVID-19 [21].