Case history and nomenclature
Benign myoclonic epilepsy syndrome in children (BMSES) was not clearly defined until it was first described in 7 young children in 1981 (Drave and Bior, 1981).
In this article, SDMED was defined as the occurrence of myoclonic seizures (MS) without seizures other than simple febrile seizures (FS) in the first three years of life in healthy children. These MPs were easily treatable and became quite rare during subsequent years of childhood. Psychomotor development remained normal, and no severe psychological consequences were observed. Since then, many other cases have been described in the literature. SDMED is included in the 1989 International Classification of Generalized Idiopathic Epilepsies (Commission, 1989). Some authors have described reflex MP in patients caused by noise or conversation, and proposed to distinguish between two separate nosological forms, and the 2nd was called reflex myoclonic epilepsy in children (Ricci et al., 1985). We think that such a division is inappropriate and will describe all cases as SDMED. To our knowledge, 98 cases have been described in the literature to date, of which 88 fit the classic description of SDMED and 10 were defined as “reflex SDMED.”
There are now 5 new patients under our supervision, four of whom have spontaneous and reflex seizures, which brings the total number of patients to 103.
The first description of SDMED indicated that the onset of the disease was before 3 years of age, while subsequent reports suggested a later onset in some patients, up to 4 years 8 months (Giovanardi-Rossi et al., 1997). This means that epilepsy of the same type can appear at different ages (Guerini et al., 1997).
General information
Juvenile myoclonic epilepsy (JME) accounts for up to 12% of all forms of this disease and about 23% of cases of idiopathic generalized epilepsy. JME is one of the types of myoclonic epilepsy - generalized epilepsy that occurs with myoclonic seizures. This group of diseases also includes: childhood benign myoclonus, West syndrome (myoclonic encephalopathy of childhood with cerebral hypsarrhythmia), Lafora disease, etc.
The first description of JME dates back to 1867. However, juvenile myoclonic epilepsy was identified as a separate nosological entity only in 1955 at the suggestion of a group of German doctors led by Janz, after which it began to be referred to as Janz syndrome. In the scientific literature on neurology and epileptology you can also find the term “impulsive petit mal”.
Juvenile myoclonic epilepsy
General provisions
Epidemiology
According to the few epidemiological data available, SDMED accounts for less than 1% of all epilepsies (St. Paul's Center, 1997), 1.3% and 1.72% of epilepsies that begin in the first year of life (Dalla Bernardina et al., 1983). , and 0.39% of epilepsies beginning in the first 6 years of life (Ohtsuka et al., 1993).
Floor
Gender distribution: 52 boys and 27 girls.
Genetics
The genetics of SDMED are unknown. There are few patients, and cases of familial SDMED have not been described. A family history of epilepsy or febrile seizures (AF) was present in 39% of 80 patients. In 70 patients, the incidence of AF in the family was 17%, and the incidence of epilepsy was 24%. It is difficult to assess the type of epilepsy found in relatives. In 10 cases it was probably idiopathic epilepsy. In the case reported by Arzimanoglou et al (1996), the proband was the 2nd of 2 brothers, and the eldest suffered from typical epilepsy with myoclonic-astatic seizures (EMAS, Douz syndrome).
Anamnesis of life
Most patients had no history of pathology before the onset of MP. Only two (1.9%) had comorbidities: Douz syndrome (Drave et al., 1992) and hyperinsulin diabetes (Colamaria et al., 1987).
However, cases of AF are not rare: 19 out of 64 patients (30%). AFs were always simple, but rare (1-2) and were observed before the onset of myoclonus and before the start of treatment (15 cases).
Reasons for appearance
JME is a severe hereditary pathology that does not develop in the case of organic, chemical or traumatic lesions. The occurrence of the disease is provoked by a genetic failure, the exact cause of which has not yet been established. There are several working hypotheses to explain the causes of the genetic failure:
- According to the first, the development of epilepsy is provoked by a defective chromosome in the structure of the q 15 gene.
- According to the second, epilepsy is caused by damage to one of the loci of the arm of chromosome 6.
- According to the third (most probable), the disease is provoked by a defect in the structure of the C6orf33 gene, as well as BRD2.
Clinical and EEG manifestations
The age at which the disease begins is usually from 4 months to 3 years. Rarely does it start earlier. Later onset was reported by Giovanardi-Rossi et al (1997) - 4 years 9 months - and Lin et al (1998) - 3 years 9 months.
At first, MPs are short, most often sparse, involving the upper limbs and head, and rarely the lower limbs. In infants they are subtle and parents sometimes find it difficult to determine their onset and frequency. They often talk about "spasms" or "head bobbing." Later, the frequency of attacks increases.
Video-EEG recordings allowed us to conduct a precise analysis of these seizures. These are more or less massive myoclonic jerks affecting the trunk and limbs, causing tilting of the head and upward and outward movement of the upper limbs with flexion of the lower limbs, and sometimes rotation of the eyeballs. Their intensity is different for each child; in the same patient it can change with each attack. In severe forms, objects suddenly fall out of the hands, and sometimes the patient falls. In moderate forms, only a short forward movement of the head or even a simple closing of the eyes is noted; as a rule, the seizures are very short (1-3 sec.), although they can last longer (in older children) and consist of pseudorhythmic twitchings that do not last. more than 5-10 sec. They develop several times a day at different intervals. Unlike childhood spasms, they do not occur in long series and do not develop at the moment of awakening, but rather during sleep. In some patients, sudden noise, sudden contact, or repeated photostimulation (RPS) can trigger seizures. With single seizures, it is difficult to assess the state of consciousness. Only with repeated seizures is there a slight disturbance of consciousness without interruption of activity. We did not observe the sudden short vocalisms reported by Lin et al. (1998). The authors point to the involvement of the diaphragm and/or abdominal muscles in the pathological process, causing expiratory noise. In reflex MP, myoclonus can be induced during both wakefulness and sleep, the excitability threshold is low in stage 1, but it gradually increases during the slow stages (Ricci et al., 1995). In patients with reflex MP, the REM sleep phase was not recorded or analyzed. Because The child’s development is normal, parents and pediatricians mistake these movements for pathology.
The EEG picture outside the attack is within normal limits.
Myoclonus is always associated with an EEG discharge. Recordings show that myoclonus is accompanied by a discharge of fast generalized spike waves (SW) or polyspike waves (PSW) with a frequency of more than 3 Hz, with the same duration as myoclonus. This is a more or less regular discharge and can begin in the frontal areas and in the vertex. Myoclonus is short (1-3 seconds) and usually isolated. The myoclonic jerk may be followed by brief atonia. Sometimes after an attack, voluntary movements appear, regarded as normal muscle contractions. And in only one patient we noted a combination of myoclonus in the deltoid muscle with pure atony of the cervical muscles. During drowsiness, myoclonus increases, but not always. They disappear during slow-wave sleep. MPs caused by tactile and auditory stimuli have the same characteristics. Ricci et al. (1995) indicate that the primary manifestations are usually, but not always, manifested only by the blink symptom, followed by 40-80 ms. followed by a myoclonic jerking of the arm. After a myoclonic attack, a refractory period begins, lasting from 20-30 seconds. up to 1-2 minutes, during which external stimuli, even fear, do not provoke attacks. Repeated photostimulation (RP) can also provoke MP (Drave et al., 1992; Todt and Müller, 1992; Giovanardgi-Rossi et al., 1997; Lin et al., 1998).
Interictal EEG data without pathology. Spontaneous SV discharges are rare; Slow waves can be detected in the central areas of the brain. PF causes SV without existing concomitant myoclonus. Short nap recordings showed normal sleep organization; generalized SV discharges may appear during REM sleep.
Medical Internet conferences
Juvenile myoclonic epilepsy (JME) was first described as a syndrome by Janz and Christian (1957) under the name Impulsive Petit Mal. JME is now considered a classic example of idiopathic generalized epilepsy. At the same time, mistakes are often made in diagnosing JME due to the fact that myoclonic seizures go unnoticed or due to incorrect interpretation of EEG results.
JME accounts for approximately 5-11% of all registered forms of epilepsy (Alfradique & Vasconcelos, 2007). Most often, the onset of the disease occurs in early or late puberty (Janz, 1985).
JME usually begins during puberty in healthy people. The first attack is often provoked by sleep disturbances and alcohol consumption. In many cases, attacks develop soon after waking up. Also, a family history often reveals the presence of epilepsy.
There are three types of seizures that can occur in JME.
1) Bilateral myoclonic seizures. They consist of single or short arrhythmic twitching of the arms, sometimes of the lower extremities, usually with clear consciousness. The jerking is so sudden that the patient may drop things he is holding. The patient and his relatives often treat these attacks not as a pathology, but rather as tremors or nervousness. If only this type of seizure exists, it often goes undiagnosed. Myoclonus can occur at different times of the day without a clear association with awakening; in some patients, only myoclonus of the eyelids occurs (Mukhin K.Yu., 2000).
2) Generalized tonic-clonic seizures (GTCS). In approximately 1/3 of cases, GTCS are the first seizures to appear, with myoclonic seizures joining later. The connection between these seizures becomes apparent in patients who experience myoclonic jerks (clonic-tonic-clonic seizure) a few seconds or minutes before GTCS. Some patients regard them as harbingers and take measures to protect themselves from harm, for example, do not leave the house, lie down, or call relatives. If the development of a full-blown convulsive attack is not preceded by myoclonus, auras do not occur.
3) Absence seizures. They can appear as the first type of attack during the development of the disease (which is significantly rare) or in the later stages of the disease.
It is rare for a patient to have only absence seizures or myoclonus; more often we are faced with a combination of all three types of seizures.
Of all patients with generalized myoclonic seizures, 90% of individuals have tonic-clonic seizures, and 30-40% have absence variants.
Juvenile myoclonic epilepsy usually responds well to therapeutic intervention (Calleja et al., 2001). However, this syndrome requires long-term treatment, since, according to observations (Delgado-Escueta & Enrile-Bacsal, 1984), about 90% of patients report a resumption of seizures after discontinuation of AED therapy.
In typical cases, no neurological or psychiatric abnormalities are detected. The patient's intellectual functions are usually not affected.
If the clinical diagnosis, based on the history, typical seizures and the absence of neurological organic abnormalities, seems clear, then there is no need for neuroimaging. It is necessary that the clinical diagnosis be confirmed by EEG. In untreated patients, interictal EEG should show typical generalized spike-wave activity with normal background activity. The hallmark of JME, however, is polyspike wave patterns, where discharges consisting of two or more spikes are followed by slow waves of high amplitude. This may be seen interictally, but should also be present if there were myoclonic jerks during the study. Myoclonus without such patterns is not compatible with the diagnosis.
If no abnormalities are found in the standard EEG, it should be repeated after sleep deprivation, and electroencephalography should be performed during sleep, since epileptiform activity increases both during sleep and on awakening (video-monitoring-EEG). Hyperventilation should increase spike-wave activity in all cases of idiopathic generalized epilepsy, and photostimulation is positive (spike-wave activity with or without clinical symptoms) in almost 50% of patients with JME. The most important part of the diagnosis is a thorough medical history, in which adolescents or young adults with new-onset seizures are specifically questioned about myoclonic jerks, their relationship to sleep deprivation, their relationship to awakening, and a description of reflex epileptic signs. Usually at least one of them is detected either clinically or by EEG examination. If the EEG reveals typical interictal, and especially seizure, signs, the diagnosis becomes indisputable.
Video-EEG usually reveals bilateral epileptiform activity. N. Usui et al. (2006) noted that 14 (54%) of 26 patients with JME had clinical or electroencephalographic focal features, or a combination of both phenomena.
One of the most pressing issues in epileptology today is the dichotomous division of epilepsies into focal and generalized. It is a widely known fact that focal forms of epilepsy often mask generalized forms due to the phenomenon of secondary bilateral synchronization (SBS) and the diffuse spread of epileptic activity with the development of seizures, which visually, based on the kinematics of the attack, can be regarded as generalized. This phenomenon is widespread in patients with symptomatic forms of epilepsy, especially in infancy and early childhood (focal “masks” of Ohtahara, West, Lennox-Gastaut syndrome, etc.), which served to isolate a special group of epileptic encephalopathies from generalized and focal forms in the project new classification of epilepsies and epileptic syndromes. Symptomatic focal forms of epilepsy are often “masked” as idiopathic forms (both focal and generalized), and often attacks, which in external characteristics resemble typical generalized ones, actually have a focal genesis (i.e., they arise due to the phenomenon of secondary bilateral synchronization with diffuse spread of epileptiform activity). This phenomenon served as the basis for defining the concept of “pseudogeneralized” seizures (Mukhin K.Yu. et al., 2006). On the other hand, the opposite fact is observed - idiopathic generalized epilepsies in a number of clinical cases have focal features in the kinematics of seizures and on the EEG, but their focal nature is excluded when using a comprehensive clinical-electro-neuroimaging diagnostic approach.
In 2000, H. Meencke raised the question that the dichotomous division of epilepsies into generalized and partial still requires evidence. From the ILAE Classification and Terminology Report (2001): “...the current concept of partial and generalized epilepsies and individual seizure types as the result of exclusively local dysfunction in one hemisphere or involvement of the entire brain is logically untenable. In particular, there may be: diffuse brain damage, multifocal anomalies, bilaterally symmetrical local anomalies... And, although the dichotomous division of epileptogenesis into partial and generalized components is still used in practice, it cannot be applied to all forms of epilepsy and all types seizures..."
In Russia, pilot studies in the field of focal features of seizures and forms of epilepsy, traditionally considered primary generalized, were conducted under the leadership of Academician V.A. Karlova. V.A. Karlov and V.V. Gnezditsky in 2005 published the results of many years of research, which showed the focal onset of absence seizure. The localization of the epileptic focus is determined in most cases in the prefrontal cortex, and it has been shown that the thalamus also plays a role in the formation of a special type of epileptic system. The generation of spikes in the facial somatosensory cortex and their subsequent propagation to the thalamus was shown in a genetic model of absence epilepsy in rats (Polack et al., 2009).
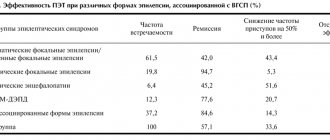
M. Koepp et al. (2005) showed that when using various diagnostic methods, signs of focal pathology in the brain are revealed (PET reveals signs of neurotransmitter dysfunction in the cerebral cortex, MRI studies demonstrate changes in the cortex of the medial frontal lobe, 1H magnetic resonance spectroscopy reveals dysfunction in the thalamus). All this suggests that in JME, to a greater extent than in other forms of IGE, the involvement of the frontal regions in the structure of epileptogenic thalamocortical “networks” plays a role, and Janz syndrome has a regional genesis with multiple foci in the frontal regions.
There are reports of the possibility of a combination of two forms of epilepsy - IGE and focal epilepsy - in one patient. A. Nicolson (2004) reports that a similar phenomenon is observed in less than 1% of patients with IGE. Zajac et al. (2007) during an MRI examination of 45 children diagnosed with primary generalized epilepsy, focal abnormalities (cysts, ventricular asymmetries, signs of focal demyelination, tumors, gliosis and atrophic processes) were found in 38% of cases. The authors recommend a more thorough search for the focal component of seizures in patients in this category. There are few studies in the literature regarding the prognosis of the course and outcome options of JME.
We analyzed the follow-up data of 40 patients with JME, observed in the neurology clinic of the Saratov State Medical University and the Road Clinical Hospital in Saratov from 1992 to 2012. The duration of observation was from 5 to 20 years.
The onset of the disease during puberty was observed in 28 patients (70%). In a small group of patients (7 people, 17.5%), the disease developed in adulthood (18-21 years). There are 3 patients (12.5%) under observation in whom the first tonic-clonic attack developed at the age of 25-30 years.
The classic combination of 3 generalized epileptic seizures in the clinical picture of the disease was recorded infrequently - 11 people (27.5%). It should be noted that absence seizures were always recorded at the onset of JME in adolescence and never occurred at the onset later. Another important clinical aspect is the complete reduction of absence seizures as the patient transitions into adulthood. This usually occurs during antiepileptic therapy or spontaneously if therapy is started later.
The combination of GTCS and myoclonic is the most common type of seizures in JME (25 people - 62.5%). And only in 4 patients (10%) JME was manifested only by myoclonic seizures and characteristic EEG changes. Isolated myclonus of the eyelids in combination with GTCS and EEG data was detected in 2 patients, and was also recorded in 4 patients in electroclinical (?) remission of JME. An adverse component at the beginning or at the end of GTCS was noted in 15 patients (37.5%), which was the reason for the diagnosis of focal epilepsy in some patients before consultation with an epileptologist. The literature describes the phenomenon of attack initiation as generalized with focal completion. Williamson R. et al. (2009), report on 6 patients who experienced attacks with a generalized onset, which later transformed into focal ones. The attack began with absence or myoclonus, after which behavioral disturbances and automatisms could be noted, and then post-attack symptoms of loss (impaired consciousness) appeared. The EEG showed generalized activity with the further appearance of regional disturbances. Interictal epileptiform activity was generalized. MRI studies revealed no pathological changes. Four patients were initially diagnosed with focal epilepsy. When antiepileptic therapy was prescribed (AEDs effective against absence seizures and myoclonus), in 3 patients the seizures stopped completely, in 3 the frequency of attacks decreased significantly.
We identified a short visual aura in front of the GTCS in the form of “fleeting” flashes and “sunrays flying at great speed” in 2 patients. The literature describes three cases of the appearance of visual auras immediately before the development of generalized tonic-clonic seizures. The results of the study showed that in idiopathic generalized epilepsy, visual auras may occur, manifested in the form of flashes of light, “lightning,” or the patient feels as if he is “seeing the sun.” In contrast to the visual auras described in occipital epilepsy, visual auras in IGE are characterized by a very short duration (Gelisse P. et al., 2008). In 2 patients, after a severe clonic-tonic attack in the early postictal period, psychomotor agitation with twilight disturbance of consciousness (focal component) developed. In the literature, there is often reference to two types of seizures that clinically have a focal manifestation - these are adversive and rotatory (torsion) seizures. The most common phenomenon is adversion of the head and eyes (in these cases a diagnosis of frontal lobe epilepsy is often made), and in cases with rotation a diagnosis of frontal or temporal lobe epilepsy may be made. Similar phenomena were reported by H. Gastaut (1986), calling this form of the disease, diagnosed in children with peak-wave discharges with a frequency of 3 Hz on the EEG, “versive epilepsy.” Many patients with similar phenomena also have typical absence seizures and myoclonic seizures. There have been reports that versive seizures before the development of GTCS can be observed at the onset of IGE, and subsequently the direction of adversion or torsion remains stable in many patients. The results of some studies have demonstrated the lack of influence of seizures with adversion or torsion on the prognosis of the disease (Aguglia U. et al., 1999).
EEG features of JME
Slow-wave changes, regional spikes or sharp waves independent of generalized discharges, regional spikes, spike-wave complexes, slow waves immediately before a generalized discharge were identified in a significant group of patients (17 people - 42.5%). As a rule, they were single, short, disappearing and/or reappearing during dynamic observation. Most often, focal EEG components were recorded during long-term EEG recordings, especially during daytime or nighttime sleep. It was noted that most often these EEG changes were recorded in patients during treatment, when remission of attacks was achieved or when drug resistance developed. In studies by CT Lombroso (1997), 32 (56%) of 58 patients with IGE had regional changes in the EEG, and at the onset of the disease, changes were observed in only 13% of patients. The author hypothesized that such patients may have either an independent cortical local pathology, or that an independent focus of epileptogenesis may form as the disease progresses. Leutmezer F. et al. (2002), on the contrary, indicated that the presence of a cortical abnormality in such cases is more likely to indicate focal epilepsy. Many researchers report the detection of regional changes in the EEG in 1/5–1/2 of patients with IGE (Panayiotopoulos CP et al., 1991; Montalenti E. et al., 2001; Aliberti V. et al., 1994; Lombroso CT, 1997).
The MRI picture in the majority of patients did not reveal organic changes in the brain (28 people - 70%). In the remaining cases (30%), MRI revealed diffuse expansion of the subarachnoid spaces, communicating hydrocephalus, internal symmetric hydrocephalus, and retrocerebellar cysts.
Monotherapy was carried out in 26 patients (65%), duotherapy in 12 people (35%) the drugs of choice were Valproate, Topirampate, Keppra, Barbiturates were used with effect. In isolated cases, especially in cases of photosensitivity, Lamotrigine was used (the likelihood of aggravation of attacks in JME was taken into account). Stable electroclinical remission was observed in 31 patients (77.5%). Long-term remission of attacks, with registration of epileptiform changes, which in some cases did not allow the abolition of anticonvulsants, was noted in 5 cases. Unfortunately, 4 (10%) patients had a drug-resistant course of the disease, which did not allow achieving long-term remission of attacks. In the best case, interictal intervals reached 8-10 months.
The fact of the appearance of epileptiform activity on the EEG in patients in remission of JME requires a careful and balanced approach. Our observations show that in the case of focal spikes, especially during sleep, it is possible to refrain from immediately returning to the prescription of anticonvulsants without high risk. Of course, this requires more frequent EEG monitoring (after 3.6 months).
remission failures.
Efficacy of therapy
JME is characterized in some cases by high sensitivity to anticonvulsants. The rate of achievement of stable electroclinical remission is 80% (32 people). In 20% of patients, it was not possible to achieve remission of attacks for more than 1-2 years, primarily this concerns relapses of myoclonic attacks. As a rule, myoclonus was provoked (sleep deprivation, stress factors, alcohol intake, a break in taking anticonvulsants for 1-2 days, etc.). The fact of a longer period of taking anticonvulsants in JME is undoubted. The longer the period of taking the drugs, the less likely it is that the attacks will relapse. As a rule, the duration of therapy to achieve electroclinical remission in JME reached at least 5-6 years. With a period of remission of 3-3.5 years, the likelihood of relapses after discontinuation of drugs increases. Of the 12 patients with remission periods of 3-3.5 years, 8 (66.6%) either had relapses of myoclonic or GTCS during the first year after discontinuation of anticonvulsants, or generalized spike-wave activity appeared during photostimulation or sleep. The appearance of such activity was considered a predictor of a high risk of developing attacks, and anticonvulsants were re-prescribed in the same dosages. A total of 12 patients (30%) in the JME group received long-term anticonvulsant medication (5 to 10 years). As mentioned above, 4 patients were pharmacoresistant. Those. the group of JME patients receiving long-term therapy consisted of 16 people (40%) due to the development of recurrent attacks, the appearance of spike-wave phenomena on the EEG or drug resistance.
In our opinion, patterns of high risk of failure of JME remission are:
- The appearance of photosensitivity discharges on the EEG, especially in the stage of clinical remission
- GTKP's "poor" response to anticonvulsants
- Polytherapy for JME
- The patient has clonic-tonic-clonic seizures (tonic-clonic seizures preceded by massive bilateral myoclonic seizures).
Atypical course of JME in adults
Previously, we (Korotkov A.G., Kabanova L.A., 2006) described 2 cases of the benign course of idiopathic generalized epilepsy in adults meeting the criteria for JME. The peculiarity of both cases is a long history of the disease with rare myoclonic seizures and a single GTCS without the prescription of anticonvulsants. Only the appearance of repeated GTCS (in 1 case, 15 years from the onset of the disease, in 2 cases, after 18 years, a recurrence in the form of a clonic-tonic-clonic attack) forced the patients to contact a neurologist and undergo an appropriate examination. After follow-up, it was possible to find out that periodically, but not more than 1-2 times a year, patients experienced morning myoclonus, more often provoked by sleep deprivation. EEG monitoring of daytime sleep in both cases revealed generalized spike wave activity, while MRI of the brain showed no organic changes. Under observation is another 36-year-old patient, who, since the age of 18, has had periods of series of myoclonus in the arms, sometimes involving the legs, with a frequency of 1 in 1-2 years. She works as a nurse, whose professional activity involves sleep deprivation. Series of myclonia up to 3-4 minutes usually occurred after night shifts in the morning hours. Repeatedly examined for this reason (routine EEG, MRI), which did not reveal pathology and twitching in the hands was regarded by the neurologist as a conversion disorder. In 2012, 2 series of myoclonus were observed after sleep deprivation, night sleep was monitored for the first time, generalized polyspike-wave activity was detected . In our opinion, the patient has a benign course of JME, which does not require the prescription of anticonvulsants at the present time.
Thus, long-term follow-up observation (from 5 to 20 years) of 40 patients with JME allowed us to come to the following conclusions:
1. JME is a syndrome of generalized epilepsy with heterogeneous clinical manifestations. In the structure of JME attacks, in some cases there are focal symptoms.
2. In the vast majority (60%) of cases with JME in adults, it is possible to achieve long-term clinical remission and discontinue anticonvulsants.
3. In the JME group, 40% of patients require long-term therapy due to drug resistance, relapse of attacks, or the appearance of spike-wave activity on the EEG after drug withdrawal.
4. The appearance of single, short bilateral (bifrontal) spike-wave discharges in remission of the disease after discontinuation of anticonvulsants does not always require a clear return to the prescription of drugs.
5. Apparently, there is a variant of the benign (atypical?) course of JME in adults with a single GTCS and extremely rare reductive provoked myoclonic seizures, which does not require the prescription of anticonvulsants.
Course and treatment
Children with SDMED do not experience seizures of any other type, even if they remain untreated (up to 8.5 years in one of our patients), this is especially true for petit epileptic or tonic seizures. Clinical examination results are normal. Interictal myoclonus was described only by Giovanardi-Rossi et al. (1997) in 6 patients. Analyzing the condition of our patients, we found mild interictal myoclonus in 2 according to EEG recordings. Many patients were not examined, but when CT and MRI were performed, the results were normal (33 patients).
Outcome likely depends on early diagnosis and treatment. Myoclonus is easily amenable to monotherapy with valproate, and the child’s development is appropriate for his age. If left untreated, the patient continues to have myoclonic seizures, which can lead to impaired psychomotor development and behavioral abnormalities.
Treatment methods were more or less thoroughly tested on 74 patients. 65 patients received monotherapy, 6 received polytherapy, and 3 received no treatment. Monotherapy included valproate (VPA), phenobarbital (PB), nitrazepam (NTZ). 6 patients received therapy with primidone (PRM) and ethosuximide (ESM). As a result of treatment, seizures disappeared in 69 patients (93%).
These data confirm that VPA is the first choice drug for SDMED. However, treatment should be carried out under the control of drug concentration in plasma, because improper use may lead to relapse or cause drug-resistant epilepsy.
Long-term results and prognosis
The duration of observation for 63 patients was 9 months. up to 27 years, of which 45 patients were observed for about 5 years. The age of patients during the observation period ranged from under 5 to over 15 years.
In all cases, MPs were stopped. The duration of the disease is known in 52 patients: in most of them, MP lasted less than one year, in 7 - from 1 to 2 years, only in 5 - more than two years.
The occurrence of generalized epileptic seizures (GES) after cessation of MP was reported in the case of 10 patients without concomitant MP.
The results of observation of 74 patients were reported.
The attacks stopped in 28 patients over the age of 6 years. In 6 children of the same age, seizures persist: with photostimulation - in 2 (Drave et al., 1992), for an unknown reason - in 3 (Giovanardi-Rossi et al., 1997). Also, attacks persisted in 13 patients under 6 years of age. 3 patients under 6 years of age remained without treatment (Ricci et al., 1995). There is no information about 24 patients.
The EEG pattern is known for 55 patients. It quickly normalized in 23. Rare spontaneous generalized SV persisted in 13. Photosensitive changes were observed in 6. Interestingly, photosensitivity can appear after the disappearance of MP and persist for many years after the cessation of MP until adulthood. Focal abnormalities were also presented. They were recorded in 5 patients during awakening as SV in two fronto-central and parietal areas, sometimes in the fronto-parietal and fronto-temporal. They disappeared over time. On the contrary, in other patients they appeared only during sleep. They still persisted at the end of the observation period in 5 of our patients, and only during sleep.
Overall, the psychological outcome was favorable and most patients recovered. This is known for sure in 69 cases. 57 (83%) were healthy, of whom 38 were aged 5 years or older. 10 (14%) had mild retardation and attended a specialist school, but none of them were admitted to hospital. In 2 patients (3%) cognitive ability was impaired and deviations in personality formation were identified: one patient had Down syndrome and severe sensitivity, the other had MP with sensitivity to IRS and to eye closure up to 5 years of age. This psychotic disorder appeared in him at the age of 10 years and progressed.
This psychological outcome depends in part on early diagnosis, allowing appropriate treatment and reassuring the family of a good future.
These data confirm the generally good prognosis of SDMED. Seizures caused by noise or conversation were easier to control than spontaneous ones. Photosensitivity was more difficult to control and persisted for several years after the seizures stopped.
Forecast
Juvenile myoclonic epilepsy is considered a chronic disease that continues throughout the patient's life. Cases of spontaneous remission are rare. In 90% of patients who, for various reasons, interrupted treatment with an antiepileptic drug, there was a resumption of epileptic attacks. However, there are indications that in some cases patients experienced long-term remission after discontinuation of the drug.
In general, with properly selected therapy, attacks are controlled in most patients, although in half of them, isolated paroxysms may be observed during treatment. A relatively treatment-resistant course is rarely observed, mainly in cases where the patient has all 3 types of JME paroxysms.
You can share your medical history of what helped you in the treatment of juvenile myoclonic epilepsy.
Differential diagnosis
If myoclonus begins in the first year of life, it must be differentiated from cryptogenic infantile seizures (CS). DS are clinically different from benign myoclonus: they are more severe and include tonic convulsions throughout the body, which is never observed in SDMED; single seizures are always combined with serial seizures in a given child; the occurrence of long serial spasms occurs after waking up. SDs then show the typical pattern of short tonic contractions on EEG, which is well described by Rusco and Vigevano (1993); Prolonged myoclonus rarely occurs. Ictal EEG variable: sudden intermittent hypsarrhythmias leveled off by superimposed fast rhythms, a large slow wave followed by leveling off or no visible change. The onset of DS is associated with behavioral changes, difficulty in speech contact, and a slowdown in psychomotor achievements.
The interictal EEG always has pathological signs: true hypsarrhythmia, or modified hypsarrhythmia, or focal disturbances; they do not detect individual or short bursts of bilateral synchronous SV or SDMED.
If psychomotor development and EEG are within normal limits on several examinations performed during wakefulness and sleep, seizures resembling DS should suggest the diagnosis of benign nonepileptic myoclonus, as described by Lombroso and Feuerman (1977). These patients even have ictal EEG without pathological changes (Drave et al., 1986; Pashats et al., 1999).
In the first year of life, myoclonic epilepsy of infancy may develop; it always begins with prolonged and frequent febrile seizures, and not with individual myoclonic seizures. In the second year, psychomotor development slows down.
If myoclonus begins after the first year of life, cryptogenic Lennox-Gastaut syndrome (CLG) may be suspected. In LGS (Byman and Dravet, 1992), the seizures are mainly not myoclonic, but myoclonic-atonic, and more often tonic, leading to sudden falls and injuries. Their EEG pattern is heterogeneous, and in the ictal period the high-amplitude either levels off, or high slow-wave activity is determined, followed by low-amplitude waves. At the very beginning, interictal EEGs may be normal, but later typical diffuse discharges of slow CO may appear. Typical electroclinical signs during sleep may be delayed in time. The diagnosis is based on the rapid association of seizures of different types, such as atypical absence seizures and axial tonic seizures, persistent behavioral disturbances and the emergence of new skills and the ineffectiveness of antiepileptic drugs.
If myoclonic seizures are isolated or associated with PEP, a diagnosis of myoclonic astatic epilepsy of early childhood (EMAI) should be considered, although in this syndrome myoclonic astanic seizures rarely begin before age 3 years (Douze, 1992). There are two significant differences: 1) the clinical aspect of seizures with stupor, which is not observed in SDMED (Guerrini et al., 1994); 2) EEG signs are also different: SV and PSV are more numerous, grouped into long flashes, connected by a typical theta rhythm over the centro-parietal zones. But some patients should probably be classified as SDMED. Similarly, Delgado-Escueta et al. (1990) included in the study or patient group, probably under the name of myoclonic epilepsy of childhood (MECD), cases of both EMAP and SDMED.
Finally, other epilepsies that begin in the first three years of life, in which myoclonus is the main seizure type and which have a variable prognosis, should be considered. They are heterogeneous: a combination of seizures of other types, persistent focal abnormalities on the EEG, delayed psychomotor development in the past, poor response to treatment, unclear prognosis (Drave et al., 1992).
Diagnostics
Confirming the presence of juvenile myoclonic epilepsy at the initial stage of its development is very difficult. Often the manifestation of JME symptoms at an early age is attributed to increased excitability and nervousness of the child, while the children themselves practically do not notice the manifestation of symptoms.
First of all, to make a diagnosis, it is necessary to exclude the possibility that the child has other types of cerebral pathologies (cancer, brain abscess, intracerebral cyst, edema, and the presence of viral and bacterial brain damage). This stage of diagnosis is not only vitally important, but also extremely difficult, since the symptoms of pathologies of this group are almost identical. To confirm the myoclonic form, MRI and angiography are used.
Further diagnostics are carried out under the guidance of an epileptologist. Under his leadership, electroencephalography is performed, which in almost 70% of cases reveals symptoms characteristic of juvenile myoclonic epilepsy.
In addition to the electroencephalogram, EEG studies (upon awakening and also falling asleep), 24-hour EEG monitoring, sleep deprivation, and photostimulation are used for early diagnosis.
Differential diagnosis
JME requires clear differentiation from other types of epilepsy that have similar symptoms. The paroxysms characteristic of JME are distinguished from the paroxysms characteristic of pathologies of other etymologies by the synchronicity of the attacks. Another important diagnostic criterion is the presence of myoclonic seizures, which practically do not occur in JME.
Also, almost all types of pathologies of the nervous system that manifest at an early age are accompanied by serious delays in mental development, which do not occur in the presence of myoclonic epilepsy.
In addition, JME is almost never complicated by seizures, in which convulsions are supplemented by absences and paroxysms that occur without loss of consciousness.
Clinical examination of the patient in order to make a diagnosis.
This examination is quite simple. It requires a careful history and repeated video-EEG recordings to demonstrate the presence of MP with generalized SV discharges, spontaneous or those promoted by drowsiness, noise, contact, or IRS. Sleep recordings can show weak activation of discharges and focal disturbances. Neuroimaging is useful to confirm the absence of brain damage, but is not necessary in the presence of typical symptoms. A neuropsychiatric examination is more useful for checking psychomotor development and tracking the course of the disease.
Treatment
VPA in monotherapy is the drug of choice, its use should be started as early as possible. It is preferable to use a solution rather than a syrup, as it is better tolerated by the child. It is necessary to strictly monitor the concentration of the drug in plasma.
A daily dose of 30 mg/kg 3 times a day is usually sufficient, but a higher dose may be required in some patients. VPA is also effective for possible febrile seizures. If VPA is ineffective, it can be used in combination with a benzoadiazepine (CLB or NTZ) or ETS and reconsider the diagnosis. Treatment should be continued for 3-4 years after the onset of the disease if it is well tolerated. In cases of purely reflex symptoms, drug therapy may not be used. If it has already started, it can be stopped abruptly, but if there is no hypersensitivity. If PEP occurs in a teenager, short-term treatment can be given at that age. Conservative treatment should be combined with psychological assistance to the family.