The cerebellum and mental disorders
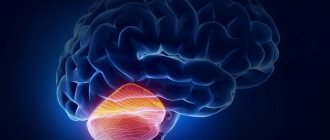
The cerebellum has long been studied exclusively for its role in motor coordination. However, research over the past two decades has shown that the cerebellum also plays a key role in many motor, cognitive and emotional processes. Several functions of the cerebellum have been described in the literature, including emotion regulation, impulsive inhibition on decision making, attention, and working memory. Many experimental studies show that the cerebellum plays a role in uncontrolled learning processes.
In addition, the cerebellum has been shown to be involved in the pathogenesis of many psychiatric disorders, including attention deficit hyperactivity disorder, autism spectrum disorder, schizophrenia, bipolar disorder, major depressive disorder, and anxiety disorders. It has been suggested that motor, cognitive, and emotional abnormalities may be caused by damage to parts of the cerebellum interacting with the motor area, prefrontal cortex, and limbic system, respectively. Some authors have suggested that the role of the cerebellum in cognitive functioning is similar to its control of goal-directed motor skills during movement.
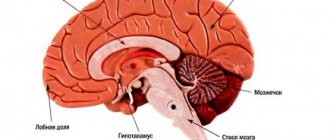
The cerebellum communicates with various brain structures and influences information processing in several brain regions, including the cerebral cortex, spinal cord, vestibular nuclei, and brainstem (eg, inferior olivary and pontine nuclei). Inputs from the spinal cord and brainstem enter the cerebellum through the inferior cerebellar peduncle. In addition, afferents from the cerebral cortex (relayed to the pontine nucleus) enter through the middle cerebellar peduncle and play a role in body balance and movement. The cerebellum "projects" into the brain and cerebral motor cortex through the red nucleus and the ventrolateral nucleus of the thalamus.
Three tracts emerge from the cerebellum: (1) the cerebellar space - its vermis, indirectly, is related to the pons and reticular formation; (2) the intermediate zone of the cerebellum goes to the red nucleus and thalamus; and (3) the lateral zone of the cerebellar hemispheres of the cerebellum extends towards the thalamus. After entering the thalamic connection, these fibers project to different parts of the cerebral cortex, including the frontal, motor, and parietal cortices. The cortico-ponto-cerebellar and cerebellar-thalamo-cortical pathways allow the cerebellum to influence information processing in cortical areas responsible for cognitive and emotional processes. These complex connections between the cerebellum and other structures may explain why damage to the cerebellum can lead to various mental disorders.
Until now, little is known about how the brain develops in ADHD patients over the course of the disorder. The researchers found volumetric abnormalities with reduced size of the brain and cerebellum that increased with age, while changes in the volume of the caudate nucleus disappeared as subjects got older. These results were found to be unrelated to psychostimulant treatment. At the same time, it has been shown that patients treated with stimulants have a larger total cerebellar volume, which exceeds the size of the cerebellum of children with ADHD who did not take stimulants. The size of the worm (vermis) may also predict treatment outcomes (smaller volumes predicted poorer results). In addition, patients with smaller worm lobules caused by stroke or other developmental abnormalities also exhibit poor concentration and environmental awareness. In general, reduction in cerebellar volume is a common finding in studies of cerebellar disorders and ADHD. However, we cannot determine whether abnormalities in the cerebellum were present at birth or developed during the child's growth and how this contributes to the etiology of ADHD.
The cerebellum is capable of influencing the motor cortex and prefrontal cortex region, two areas that are responsible for motor control and social cognition, so it is not surprising that abnormalities in the cerebellum can cause the symptoms seen in autism spectrum disorder (ASD). Decreased Purkinje cell activity results in ASD-like behaviors, including abnormal social and motor behaviors. Postmortem studies have also shown decreased Purkinje cell density in patients with ASD. Being gamkergic, Purkinje cells influence the activity of the cerebellum-cortex tract, which may explain the occurrence of stereotypic (repetitive) movements in ASD. This, however, needs to be confirmed or unconfirmed in studies that link Purkinje cell loss to specific symptom domains (motor and social dysfunction) in ASD. Reduced activity in the cerebellar peduncles is also associated with poorer motor abilities in patients with ASD. There is an assumption of an additional defect in the formation of networks of neurons in the cerebellum and frontal lobe in Asperger syndrome. These deficits may be responsible for the motor and cognitive impairments observed in patients with ASD. Impaired adaptation of social behavior in patients with ASD may be caused by disruption of feedback pathways from the cerebellum to the cerebral cortex. In addition, abnormally organized fibers of the middle and inferior cerebellar peduncles (peduncles) connecting the cerebellum to the frontal lobe were identified. This may be either a direct cause or a consequence of changes in the cerebral cortex and cerebellar nuclei in patients with autism. These pathological changes explain the coordination deficits and ataxia in some forms of autism. Thus, there are currently three main cerebellar abnormalities observed in patients with ASD: reduced Purkinje cell size, decreased cerebellar volume, and disruptions in feedback between the cerebellum and cortical areas. Because Purkinje cells are inhibitory in nature, a deficiency of these cells reduces the inhibition that the cerebellum sends to cortical and subcortical regions, resulting in the hypersensitivity of these brain regions found in most children with autism.
Most research to date has focused on Purkinje cells in ASD, particularly Asperger's syndrome or autism; however, it would be useful to understand how Purkinje cell density relates to autism severity. Because Purkinje cells inhibit areas of the cerebral cortex and midbrain areas, it can be hypothesized that patients with full-blown autism would also have a much lower density of Purkinje cells.
Structural brain imaging studies have revealed decreased cerebellar volume in patients with schizophrenia, including decreased volume of the cerebellar vermis. Changes in cerebellar volume in patients with schizophrenia were associated with: behavioral disorders arising in the perinatal period; male gender, early onset and manifestation of schizophrenia, chronic course of the disease and clinical picture with predominantly positive symptoms. Functional brain imaging studies in patients with schizophrenia indicate decreased blood flow in the cerebral cortex and cerebellar vermis during many cognitive tasks of attention, memory, including both short-term and working memory tasks, and social performance.
Research regarding the role of the cerebellum in the motor side effects observed in patients with schizophrenia treated with antipsychotic drugs is limited. For example, one study showed decreased cerebellar activity in schizophrenia patients with akathesia symptoms during treatment with olanzapine, but it is not known how changes in cerebellar function might lead to this complication of antipsychotic therapy. The smaller cerebellar volume in ASD may be explained by a decrease in the number of Purkinje cells; however, the number of Purkinje cells does not differ between healthy individuals and patients with schizophrenia. This means that the decrease in cerebellar volume in schizophrenia may be due to the reduction or absence of various parts of the cerebellum.
Many studies demonstrate cerebellar changes with decreased cerebellar volume and atrophy in patients with bipolar disorder. Interestingly, the volume of the V3 red subregion of the cerebellum is significantly reduced in patients with multiple episodes of bipolar disorder compared with data obtained from healthy controls, while the volume of the V2 red subregion is smaller in patients with multiple episodes than in patients with first episode. It was also found that the decrease in cerebellar volume was greater in patients with infrequent drug use compared to patients on active drug treatment for a long period of time. In a study using functional MRI in patients with BD, increased glucose metabolism was found in the cerebellum of patients who were treatment resistant. However, it is unclear whether these changes in cerebral blood flow and metabolism are primary or secondary to BD. Due to the inhibitory nature of cerebellar activity, activation of this structure can be expected to decrease during manic phases and increase during depressive phases. Alternatively, activation from the cerebellum may remain constant while the rest of the brain operates on autopilot, attempting to compensate for the aberrant inhibitory activation originating from the cerebellum.
Patients with major depressive disorder (MDD) also showed various abnormalities in the cerebellum. Yucel et al. (2013) found a significantly smaller region of the vermis known to regulate emotion and cognition in patients with MDD. Similar to bipolar disorder, studies have also reported smaller cerebellums in patients with MDD. Severely depressed patients treated with antidepressant drugs showed increased cerebellar activity and increased blood flow in the vermis, compared with patients in remission or healthy subjects. These findings were positively correlated with the severity of depressive episodes, the severity of cognitive deficits, and antidepressant resistance. Abnormal connections between the cerebellum and the frontal lobe have also been found in patients with severe depression who were resistant to treatment, and there are similar reports of depression in the elderly. In general, studies of the cerebellum in MDD have shown decreased cerebellar volume, increased cerebellar activity, and impaired connections to the cerebral cortex. The reduction in cerebellar size is an interesting phenomenon because it is also present in patients with ADHD. Moreover, this cerebellar contraction, typical of both patient groups, appears to be focused on the vermis, an area that is heavily involved in attention-related processes that is also impaired in patients with MDD. It is interesting to note that this region is also impaired in bipolar patients who have attention deficits. Some studies have found that changes in cerebellar activity are not associated with mood changes in MDD.
The literature reports cerebellar dysfunction in anxiety disorders, which may be associated with increased arousal in post-traumatic stress disorder (PTSR), generalized anxiety disorder (GAD), and social anxiety disorder (SAD).
A study using single photon emission computed tomography (SPECT) by Bonne et al. (2003) found increased cerebellar activity during repeated traumatic events in patients with post-traumatic stress disorder (PTSR). Cerebellar hypersensitivity was positively correlated with elevated blood pressure and heart rate, suggesting a possible role for the cerebellum in the regulation of sympathetic activity, which may explain its role in the pathogenesis of anxiety disorders. Studies of patients with panic disorder have found significant changes in glucose metabolic levels in the pons, midbrain, thalamus, hippocampus, amygdala, and cerebellum.
The cerebellum has been found to be associated not only with psychiatric and cognitive symptoms in various psychiatric and neurological disorders, but also with features of pharmacological and cognitive behavioral therapy. However, it is still unclear how cerebellar dysfunction is associated with various symptoms in psychiatric disorders. Most research findings are inconclusive when considering the specific anatomical abnormalities in the cerebellum that are present in psychiatric disorders. However, some of the disorders discussed with similar cerebellar disorders, such as ASD, schizophrenia, bipolar, and MDD, show reduced volume in the vermis region; however, their symptoms differ significantly from each other. Because each region of the cerebellum projects its influence to different areas of the cerebral cortex and midbrain structures, the variety of symptoms indicates that abnormalities in each disorder are concentrated in specific regions rather than the cerebellum as a whole. This may explain the wide range of symptoms observed in disorders identified in the cerebellum. For example, a strong connection between vbis areas VIIb and IX and the visual network was noted by Sang et al (2012). This region is also known to influence blood flow in patients with schizophrenia, which in turn may be a factor associated with visual hallucinations. The same can be said for connections to hemispheres VI, VIIb and VIII, which show connections to the “auditory network” of neurons and may explain the auditory hallucinations experienced by some patients with schizophrenia.
Material and research methods
The studied sample consisted of 30 patients with schizophrenia aged 18-30 years, whose disease duration did not exceed 3 years. The examination was carried out upon admission to the clinic before the start of drug treatment.
Neuropsychological research methods included a special scale for assessing the functional pathology of the cerebellum in schizophrenia, created on the basis of the method developed by Zueva under the guidance of Korsakov (2), as well as methods from the classical Luria examination scheme. The latter included Head tests, sensitized techniques for assessing agnosia (crossed out images), superimposed Poppelreiter figures, memorization of 5 difficult-to-verbalize figures, memorization of material unstructured in meaning (“10 words”), tests for dynamic praxis. Speech, neurodynamic parameters of activity, programming, regulation and control of activity were assessed.