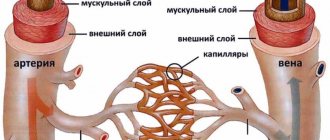
Dementia with Lewy bodies (DLB) is a progressive neurodegenerative disease, most often onset in old age and manifested by increasing cognitive decline, parkinsonian symptoms, psychotic disorders and autonomic dysfunction. The true scientific history of DTL begins in the late 70s of the last century. It is associated with the name of the Japanese researcher K. Kosaka, who described several patients with dementia in whom Lewy bodies were found in the cerebral cortex at autopsy. In connection with DLB, one cannot help but recall F. Lewy himself, the famous German pathologist, who in the 30s of the last century, fleeing the Nazis, moved to the USA. He made the main discovery of his life in 1912 at the age of 27, working in Breslau (Wroclaw), describing eosinophilic inclusions in brain neurons in patients with Parkinson's disease. F. Lewy discovered intracellular inclusions in the motor nucleus of the vagus nerve and Meynert's nucleus, but not in the substantia nigra, the lesion of which is currently associated with the development of major motor disorders. This task was completed by another young scientist - our compatriot K.N. Tretyakov, who in 1917, while on an internship at the Salpêtrière clinic in Paris, described similar inclusions in the cells of the substantia nigra and proposed calling them “Lewy bodies.”
Etiology
As with AD3, most cases of DLB are sporadic; however, it appears that familial cases are more common in DLB than in AD or vascular dementia. Approximately half of patients with DLB have one or more first-degree relatives with dementia or parkinsonism. In familial cases of DLB, clinical polymorphism is observed, which complicates medical genetic analysis. Only in a relatively small proportion of familial cases of DLB, genetic mutations were identified: triplication of the synuclein gene on chromosome 4, mutations in the LRRK2 gene on chromosome 12, gene changes on the short arm of chromosome 2 (2q35-36), the functional purpose of which remains unclear.
Astrocytes - a possible cure?
Recently, several scientific papers have been published on the transformation of various cells into dopaminergic neurons. Basically, the researchers chose astrocytes - cells that support the vital activity of neurons. They got their name from their characteristic star-shaped shape (Fig. 2). The responsibilities of these cells include providing the metabolic needs of neurons, participating in the timely release of neurotransmitters from nerve endings, storing nutrients and regulating neuronal activity.
Figure 2. Auxiliary cells of the nervous system - astrocytes. Normally, they ensure the vital activity of neurons, but by influencing the molecular processes occurring in astrocytes, they can be “transformed” into neurons of any type.
Human Astrocytes
But most of all scientists are interested in their reparative function. In 2014, a group of scientists from Lund University and Karolinska Institutet discovered that when nerve tissue is damaged after a stroke, astrocytes are able to replace dead neurons [12]. At the same time, the Notch1 signaling pathway, which is of key importance in the processes of cell proliferation and differentiation, “turns off” in them. In a healthy brain, this pathway is active and blocks the conversion of astrocytes into neurons. However, after a stroke, this mechanism is suppressed, and astrocytes can begin to transform into neurons.
The idea for the study, conducted by a team from the University of California, is not new. Back in 2021, a group of Swedish scientists turned human astrocytes into neurons in vitro using viral vectors. After this, mouse astrocytes were also transformed, but in vivo. For reprogramming, they used three transcription factors (NEUROD1, ASCL1 and LMX1A) and miR218 microRNA [13]. Astrocyte transformation in vitro has been improved using molecules capable of inducing chromatin rearrangement and activating several signaling pathways.
However, a 2021 study proposes a much simpler method for reprogramming astrocytes: simply blocking the production of a single protein.
Neuromorphology
Macroscopically, in most patients with DLB, as in many other neurodegenerative diseases, diffuse brain atrophy is detected with expansion of the cortical grooves and lateral ventricles, although on average it is less pronounced than in AD.
The development of dementia in DLB is associated with a degenerative process in the neocortex and limbic cortex, primarily in the anterior cingulate, parahippocampal gyrus, frontal and parietal cortices. As modern methods of functional neuroimaging show, already at an early stage there is a decrease in the function of the occipital cortex, which can serve as one of the differential diagnostic signs distinguishing DLB from AD. At the same time, atrophy and microscopic signs of degeneration, including Lewy bodies, in the occipital cortex are very moderately expressed. Thus, dysfunction of the occipital cortex, which may be associated with the early development of visuospatial impairment, appears to be associated with impaired neurochemical processes (eg, cholinergic denervation). The development of parkinsonism, observed in the majority of patients with DLB, is associated with the involvement of the substantia nigra and striatum in the degenerative process, and sleep disorders with damage to brain stem structures. The development of autonomic dysfunction is explained both by damage to the stem nuclei and by the involvement of peripheral autonomic structures, which already at an early stage of the disease leads to autonomic denervation of the heart.
1.General information
The term “dementia” is used both in neurology and in psychiatry, and, strictly speaking, it has more psychiatric meaning: a full literal translation means something like “deprivation of reason”, “insanity” (and not “madness”, as is most often translated ). This refers to a state or process, depending on the context, of the disintegration of cognitive functions (memory, attention, recognition, etc.), degradation of speech in composition and structural organization, and a progressive decrease in the ability to abstract and draw logical conclusions. However, in neurology, along with this general meaning, dementia is also understood as a nosological category of clearly defined neurodegenerative diseases. The essence of such diseases, no matter what they are caused by, is a gradual simplification, primitivization, loss of volume and functional consistency and, ultimately, the death of brain neural tissue at the cellular level - which is implied by the related concepts “dystrophy, degeneration, atrophy” with the prefix “neuro-”.
The most famous neurodegenerative disease (at least, it is most often mentioned by non-specialists, and as a collective name for all age-related dementias in general) can be considered Alzheimer's disease. The second or, according to other sources, the third most common organic neurodystrophy is called “dementia with Lewy bodies” (DLB). Etiopathogenetically and symptomatically, it is indeed associated with both Alzheimer's disease and another frequently mentioned neurodegeneration - Parkinson's disease. However, dementia with Lewy bodies was singled out as a separate diagnosis at the end of the twentieth century because it has not only similarities, but also a number of distinctive features in the clinic, course, pathomorphology and prognosis.
A must read! Help with treatment and hospitalization!
Clinic
The range of clinical manifestations of DLB is very wide and includes cognitive, psychotic, motor disorders, autonomic dysfunction, sleep and wakefulness disorders. Cognitive decline is an obligate manifestation of DLB. Most cases of DLB are characterized by a unique neuropsychological profile, in which subcortical and cortical disorders are intertwined. At the early stage, disorders of the neurodynamic and regulatory type dominate, the leading of which are attention disorders, and both concentration and stability of attention suffer. DLB is also characterized by the early and rapid development of visuospatial impairments, which are one of the main “calling cards” of the disease.
At first, difficulties in performing tests are explained by a violation of attention, planning and execution of complex constructive tasks, and only partly gnosis. But as the disease progresses, disorders associated with dysfunction of visual associative pathways quickly appear. Over time, errors in visual recognition of objects, familiar objects, and finally close relatives appear. Moreover, self-identification also suffers - seeing his reflection in the mirror, the patient (usually in the advanced stages of DLB) mistakes him for another person (mirror symptom). Violation of regulatory (frontal) and visuospatial functions is one of the main prerequisites for the early appearance of visual hallucinations. In this case, memory remains more intact and is characterized not by a defect in memorization, consolidation and storage, but rather by a violation of the search and reproduction of traces, which is associated with dysfunction of the frontal parts and the relative intactness of the medial parts of the temporal lobes. Often, relatives are surprised to hear from a patient in moments of enlightenment a story about the events of a past life with details that they themselves have long forgotten.
Another characteristic feature of the mental status is fluctuations, characterized by transient episodes of decreased attention and activity with unspontaneity, unresponsiveness, and sometimes confusion. Such episodes of deterioration can last from several minutes to several hours and are often mistakenly regarded as manifestations of cerebrovascular insufficiency or as an epileptic phenomenon. Fluctuations in mental status also occur in other forms of dementia - vascular dementia or AD, but only in DLB they occur at an early stage of the disease. With DLB, longer-term fluctuations often occur, characterized by the presence of “bad” and “good” days (“second-order” fluctuations) or multi-day episodes of decompensation of the main manifestations of the disease (“third-order” fluctuations). Episodes of decompensation are often the result of inadequate therapy, dehydration, intercurrent illnesses, overheating, but sometimes they also occur spontaneously. In severe cases, they can result in death; in milder cases, they can result in restoration of the previous functional state. But most often, recovery is partial, which moves the patient “one step higher” in the progressive course of the disease.
Psychotic disorders are another typical, diagnostically important feature of DLB, which often has a dramatic impact on the subsequent course of the disease. Sometimes visual hallucinations, which are considered the most characteristic variant of psychotic disorders in DLB, appear already in the prodromal phase of the disease, when there is still no disturbance in daily activities that allows one to diagnose dementia. The spectrum of psychotic disorders in DLB includes, along with visual hallucinations, the so-called extracampal phenomena (feelings of the presence of a stranger or someone passing by), hallucinations of other modalities (auditory, tactile, somatic), illusions, delusional syndromes (often related in content to hallucinations). ), identification disorder syndromes (for example, Capgras syndrome), delirium.
The spectrum of psychopathological disorders should be supplemented by anxiety and depression, which often appear already at the prodromal stage of the disease, but persist at later stages, as well as such conditions as apathy, agitation, disinhibition, which, as a rule, appear at a later stage of the disease, significantly complicating nursing.
Motor disorders in DLB are characterized by symptoms of parkinsonism. In the early stages, extrapyramidal pathology occurs only in 50% of patients and is often represented by relatively mild symptoms. This often misleads a neurologist if he superficially examined the patient and did not pay attention to pronounced cognitive and neuropsychological disorders. As a result, he mistakenly diagnoses the early stage of PD and, in accordance with this, prescribes dopamine agonists, anticholinergics, etc., which can provoke psychotic disorders in this category of patients. Next, the patient is transferred to a psychiatric institution, where he is prescribed antipsychotics for psychosis, the use of which even in minimal doses in patients with DLB can lead to a sharp deterioration of the condition such as an akinetic crisis, ending in death in at least a third of patients.
As the disease progresses, the incidence of extrapyramidal symptoms increases to 80%. Parkinsonism in DLB is characterized by a more frequent left-sided onset, early development of axial disorders (hypomimia, postural instability with frequent falls, early walking impairment with freezing, truncal bradykinesia, camptocormia, dysphonia or dysarthria). Disturbances in sleep and wakefulness can manifest themselves in patients with DLB long before the appearance of other symptoms of the disease. This primarily refers to behavioral disorder (“psychomotor agitation”) during the rapid eye movement sleep phase. Such disturbances may precede other manifestations of the disease by several decades. Another common symptom is daytime sleepiness.
The early development of autonomic dysfunction is another of the most characteristic manifestations of DLB, associated with damage to the stem autonomic structures, the lateral horns of the spinal cord, and the peripheral autonomic system. The most common autonomic manifestations are neurocardiovascular instability with orthostatic hypotension and a tendency to faint, neurogenic urinary disorders (frequent imperative urination, nocturia, subsequent partial urinary retention or incontinence), gastrointestinal dysfunction (gastroparesis, constipation or diarrhea).
Symptomatic manifestations
At the initial stage, the disease practically does not manifest itself. It can be asymptomatic for 5 years. Among the main list of signs of DLB are:
- mental lack of necessary adaptation mechanisms in order to adapt to conditions dictated from the outside;
- instability while walking;
- unstable psychological state;
- speech disorder;
- stiffness, retardation of movements;
- hallucinations;
- muscle stiffness;
- sensitivity to neuroliptics;
- disorder of reasoning, judgment;
- sweating;
- paranoid;
- tremor of the limbs.
If at least one of the listed symptoms is observed, you should urgently consult a doctor. Timely detection of pathology and its therapy have a more favorable prognosis.
Diagnostics
Today, diagnosis is based on the analysis of the entire complex of clinical signs and the results of neuropsychological testing, taking into account, in some cases, the results of instrumental research methods. In favor of DLB, in the presence of a characteristic clinical picture, is the absence of pronounced vascular or other changes on CT or MRI that better explain the clinical picture.
In this case, moderate or severe diffuse cerebral atrophy with a few lacunar foci and/or moderate periventricular leukoaraiosis is allowed. The severity of cerebral atrophy and especially hippocampal atrophy in DLB may be significantly lower than in AD, even with comparable severity of dementia.
Competition “Bio/Mol/Text”-2020/2021
This work was published in the “Free Topic” category of the “Bio/Mol/Text” competition 2020/2021.
The general partner of the competition is the annual biotechnology conference BiotechClub, organized by the international innovative biotechnology company BIOCAD.
The sponsor of the competition is SkyGen: a leading distributor of life science products on the Russian market.
Competition sponsor: the largest supplier of equipment, reagents and consumables for biological research and production.
"Book" sponsor of the competition - "Alpina Non-Fiction"
Disclaimer
Biomolecule doesn't usually publish papers on the same topics, but this year we accepted two papers based on the same 2021 Nature publication. The article you are reading is a review article, but here is its news counterpart [16].
Parkinson's disease is the second most common neurodegenerative disorder. It is characterized by the death of neurons in the substantia nigra of the brain. Current therapy aims to limit disease control. There is still no effective cure. A recent study may radically change this deplorable situation. Hao Qian from the University of California, San Diego (USA), together with colleagues from Peking University (China), demonstrated the successful conversion of astrocytes into neurons in situ in a mouse model of Parkinson's disease. However, let's start with the general characteristics of this disease.
Mechanism of astrocyte transformation
PTB1 as a target for astrocyte reprogramming [14]. This protein is synthesized in astrocytes and inhibits differentiation into neurons. Decreased production of PTB1 causes the production of its neural variant nPTB1 .
First, the scientists transformed astrocytes in vitro. Astrocytes were isolated from mouse midbrain and cortex and from human cortex. The researchers used a hairpin RNA to the Ptbp1 gene, which encodes the PTB1 protein. Hairpin RNAs work on the principle of RNA interference: they interact with the messenger RNA of a specific gene and cause its degradation, resulting in the cell's inability to produce protein. The level of PTB1 in astrocytes dropped, which led to the transformation of astrocytes into neurons.
The researchers then moved on to in vivo experiments: They depleted the PTB1 protein in the mouse brain. Symptoms of Parkinson's disease in mice were induced using a toxic dopamine analogue, 6-OHDA, which causes the death of dopaminergic neurons. Qian and colleagues used transgenic mice producing cre recombinase. This allowed the viral vector to be targeted directly to astrocytes. To make sure that the virus reached its target, a red fluorescent protein gene was inserted into it. The viral vector contained a small hairpin RNA (shPTB) that blocked the Ptbp1 gene. This strategy also led to the conversion of astrocytes into neuronal cells and the restoration of motor activity (Fig. 3).
Figure 3. Left: Mouse astrocytes. Right: neurons obtained through astrocyte reprogramming.
One-Time Treatment Generates New Neurons, Eliminates Parkinson's Disease in Mice