Acoustic neuroma - (vestibular schwannoma, acoustic neuroma) are benign, slow-growing brain tumors that have a chronic course depending on the chosen treatment method and clinical manifestation.
Based on the reason for their occurrence and the mechanism of development, two types of acoustic neuromas are distinguished:
- sporadic acoustic neuromas – etiology is unclear;
- neurofibromas – arising from neurofibromatosis types I and II. The development of the disease is associated with damage/mutation of the NF2 gene on the long arm of chromosome 22 (22q12).
According to existing registers in the Russian Federation, 1800-2000 sporadic acoustic neuromas are diagnosed per year.
General information
Schwannoma (neurinoma) is a neoplasm of Schwann cells, which are a component of the myelin sheath of nerves.
Schwannoma can have different localizations, affecting the spinal roots, peripheral nerve trunks of the extremities, cranial nerves (facial, trigeminal, vagus, glossopharyngeal), and less commonly, the nerves of the stomach, intestines, and pharynx. Among verified brain tumors it accounts for 8-10%, among spinal cord tumors about 20%, among neoplasias of peripheral nerves it occurs in almost 50% (for example, Morton's neuroma). Among the lesions of peripheral nerves, the most common is Morton's neuroma (foot), which is characterized by fibrous thickening (growth) of the tissues of the foot around the plantar nerve. Among brain tumors, the most common is acoustic neuroma (damage to the vestibulocochlear nerve). The vestibulocochlear (auditory) nerve consists of an auditory part, which transmits signals from auditory receptors to the cerebral centers, and a vestibular part, which carries signals from the vestibular receptors of the cochlea.
The frequency of occurrence according to different authors varies between 0.6-1.9/100 thousand population. The tumor is predominantly benign and develops slowly over many years. Malignant schwannoma is a fairly rare phenomenon, occurring in 4-7% of all cases. A neuroma of the brain itself is not dangerous, but over time, as it grows and increases in size, adjacent structures are compressed.
More often (1.5-2 times) the disease occurs in women. The tumor can form in people of any age (usually 30-40 years old). The disease does not occur in children before the onset of puberty. Neuromas are not caused by diseases of the central nervous system, occur sporadically, and are not inherited. Acoustic neuroma is unilateral in the vast majority of cases. Bilateral neuromas account for only 4% of cases (in patients with neurofibromatosis type II).
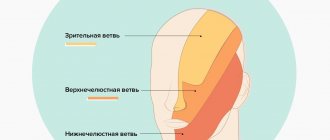
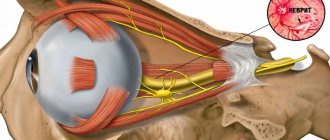
The most common location of the tumor is at the entrance to the internal auditory canal at the end of the auditory nerve. Since the auditory nerve, together with the vestibular and motor facial nerves, enter a single internal auditory canal at the base of the skull, the enlargement of the tumor compresses all adjacent nerves, causing typical clinical symptoms (Fig. below).
In most cases, the tumor grows slowly. Its growth rate varies between 1-10 mm/year and, as the neuroma grows, it spreads from the internal auditory canal to the cerebellopontine angle. In the absence of timely and adequate treatment, there is a significant risk of loss of hearing, taste perception, vision and various cerebral complications, as evidenced, among other things, by reviews of patients visiting a specialized forum.
Methods for diagnosing neuroma
The list of studies is selected by a neurosurgeon or neurologist. Diagnosis begins with excluding diseases with similar symptoms, a physical examination is carried out - examination, questioning the patient for complaints.
The following examinations may be prescribed:
- For intracranial schwannoma - MRI or CT scan of the brain. CT is less informative because it does not “see” tumors smaller than 2 cm. If MRI cannot be done, then CT with contrast is done.
- MRI or CT scan of the spine. Allows you to detect tumors compressing the spinal cord and spinal nerve roots.
- Audiometry. This is part of a comprehensive diagnosis of auditory nerve schwannoma. It makes it possible to find out the degree of hearing loss and the reason for its decline.
- Ultrasound or MRI if the tumor is located on the peripheral nerves. The first reveals thickening of the neurilemma. MRI determines the exact location of the tumor, its structure and the extent of damage to the nerve fiber.
- Electroneuromyography. In this way, the passage of electrical impulses along the nerve is assessed. It is used for almost any type of pathology and assesses the degree of disruption of the nerve structure.
- Biopsy. This is a lifetime collection of biomaterial followed by cytological analysis. Determines whether a tumor is malignant or benign.
Pathogenesis
Neuroma grows from the vestibular membrane (Schwann cells) of the 8th nerve. Schwann cells are auxiliary cells of the nervous tissue that act as a scaffold for the axons of the peripheral nerve. Tumor growth can occur both in the direction of the auditory canal and in the direction of the cerebellopontine angle. Accordingly, depending on the direction of tumor growth/its size, it can compress the V and VII cranial nerves, the pons, the cerebellum, and the caudal group of cranial nerves.
When growing into the cerebellopontine angle after reaching a size of 1.5 cm in diameter, it begins to influence (compress) the lateral surfaces of the brain stem, causing a displacement to the contralateral side of the brain stem. When the tumor reaches a size of 2 cm, it exerts pressure in the upward/forward direction, causing compression of the trigeminal nerve, which is manifested by impaired sensitivity on the corresponding half of the face/decreased corneal reflex. A tumor in the cerebellopontine angle with a size of 4 cm affects the IV ventricle/Sylvian aqueduct with the gradual development of occlusive hydrocephalus.
Macroscopically, a neuroma looks like a tuberous formation of irregular shape, surrounded by a yellowish capsule. The tumor, as a rule, does not grow into the surrounding tissue; cysts may be visible on the incision. The photo above shows a macroscopic view of an acoustic neuroma.
Causes of malignant neuroma
The exact causes of the disease have not yet been clarified. It is believed that the appearance of malignant neuroma is promoted by:
- burdened heredity;
- ionizing radiation (the latent period after exposure to this factor can last up to 15 years);
- contact with asbestos or wood preservatives;
- immunodeficiency;
- benign neuroma (but malignancy is rare);
- ganglioneuroma (but degeneration is rare);
- neurofibromatosis (but malignancy is rare).
Classification
The classification of neuroma is based on the size/position of the tumor relative to the internal auditory canal and brain stem, according to which there are several classifications.
W. Koos classification (stages of tumor growth):
- Stage I - the neuroma does not extend beyond the internal auditory canal, the size of the tumor does not exceed 10 mm;
- Stage II - neuroma extends into the cerebellopontine angle, tumor size up to 20 mm;
- Stage III - the neuroma reaches the brain stem, but does not compress it, the tumor size is up to 30 mm;
- Stage IV - the neuroma compresses the brain stem, the size of the tumor exceeds 30 mm.
Classification according to M. Samii (neurinoma stages):
- T1—neurinoma is located directly in the internal auditory canal;
- T2 - neuroma grows from the internal auditory canal;
- T3a - neuroma fills the cistern of the cerebellopontine angle;
- T3b - neuroma reaches the brain stem;
- T4a - neuroma compresses the brain stem;
- T4b - neuroma grossly deforms the 4th ventricle/brain stem.
There are also unilateral and bilateral acoustic neuromas.
Types of extracerebral formations of the brain
A feature of extracerebral formations is their long course without obvious clinical manifestations. Patients are unaware of the development of the disease for a long time and do not seek medical help, until the pathological focus increases significantly. Often it is an accidental discovery during a planned preventive examination.
Meningiomas are characterized by relatively slow growth and limited location. The pathological focus develops from the arachnoendothelial cells of the meninges. Statistics show that the disease occurs mainly in women over 30 years of age. Such extracerebral formations of the brain can reach significant sizes.
Most often they are localized in the area of the cranial fossa. In this case, the patient’s sense of smell deteriorates, various mental disorders and decreased vision are possible. With an extracerebral space-occupying lesion in the parietal region, compression of the optic nerves is possible.
Symptoms
Symptoms of acoustic neuroma are caused by several processes:
- damage to the cranial nerves;
- compression of cerebral vessels;
- compression/displacement of the brain stem;
- compression of the 4th ventricle.
The clinical picture is determined by the size/localization of the tumor and can be represented by the following syndromes:
Cochleovestibular syndrome . It is characterized by hearing disorders and symptoms of damage to the vestibular apparatus. Initially, symptoms of irritation of the VIII nerve appear, manifested by a “characteristic” noise in the form of a whistle, ringing, or surf noise, which appears long (within several years) before the development of intracranial hypertension . The noise corresponds to the location of the tumor. The phenomena of loss gradually develop: first, partial deafness in high tones develops, and later complete loss of hearing/bone conduction from the side of the tumor localization. In 60% of patients, symptoms of damage to the vestibular nerve (disorder of the vestibular system) appear, which is manifested by a feeling of instability ( dizziness ) with sudden turns of the body/head, spontaneous nystagmus and loss of vestibular excitability. Patients may complain of pain in the back of the head on the side of the tumor, radiating to the neck.
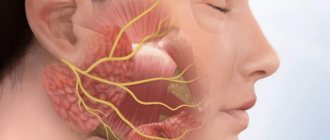
Cranial nerve compression syndromes . Most often, compression of the facial nerve is observed initially. Despite the fact that the facial nerve suffers relatively little compared to distant nerves, its compression is manifested on the affected side by mild insufficiency/paresis of its branches, much less often by spasm of the facial muscles.
On the part of the facial nerve, relatively severe disturbances develop when the neuroma is localized in the internal auditory canal, when the facial nerve, together with its intermediate portion, is strongly compressed, which is expressed in impaired salivation on the affected side/loss of taste in the anterior 2/3 of the tongue. Often there are changes in the trigeminal nerve: hypoesthesia in the nasal cavity/weakening of the corneal reflex on the side of the tumor. At a late stage, hypoesthesia in the area of 1-2 branches (changes in skin sensitivity) and absence of the corneal reflex may be noted. Motor disturbances may be present in the form of atrophy of the masticatory muscles, which manifests itself when opening the mouth by deviation of the lower jaw towards paralysis.
Next in frequency of lesions are the glossopharyngeal/abducens nerves. Disturbances from the abducens nerve are manifested by transient diplopia and failure to reach the external commissure of the eyelid of the edge of the iris when the eye is abducted towards the tumor, and from the glossopharyngeal nerve - a complete absence/reduction of taste in the area of the posterior third of the tongue. Disorders of the XI and XII pairs of the cranial nerves (cranial nerves) are much less common. As a rule, cranial nerve disorders are characteristic of tumors growing in a caudal direction or with large tumor sizes. It is large in size. Unilateral paresis of the hypoglossal nerve (XII) is expressed in deviation of the tip/atrophy of the muscles of the corresponding half of the tongue. When the vagus nerve is damaged, unilateral paresis of the soft palate/vocal cords with impaired swallowing and phonation is observed.
Brain stem compression syndrome . In cases of medial tumor growth, disorders of the brain stem and the corresponding half of the cerebellum develop simultaneously. Symptoms of disruption of the brain stem pathways (pyramidal symptoms) are mild and often paradoxical (observed on the side of the tumor, not contralateral), which is due to a stronger influence on the pathways of the opposite pyramid of the temporal bone. This manifests itself in the fact that it is weakly expressed, and since it has more than the tumor itself. Sensitivity disorders are extremely rare.
Cerebellar compression syndrome . The symptoms of cerebellar compression are caused by loss of functions of the compressed hemisphere/conduction disturbances of the compressed cerebellar peduncle. Symptoms of cerebellar disorders appear on the side of the tumor in the form of slowness of movements/ hypotonia of the muscles of the extremities, ataxia , missed movements when performing knee-heel/toe-nose tests, deviation towards the affected cerebellar hemisphere in the Romberg position, spontaneous nystagmus on the side of the tumor.
Intracranial hypertension . Symptoms of intracranial hypertension include headaches, especially in the morning after waking up, congestive optic discs, and vomiting.
The features of the clinical picture are largely determined by the direction of tumor growth: for lateral neuromas, the growth of which occurs mainly in the internal auditory canal, the following are characteristic: early loss of hearing, vestibular function, and taste on the side of the tumor on the anterior 2/3 of the tongue. More often, more pronounced peripheral paresis of the facial nerve . Much later develops: fundus congestion/intracranial hypertension. Tumors with a mediooral growth direction are characterized by early onset of brainstem symptoms/increased intracranial pressure. Caudal tumor growth is characterized by severe early dysfunction of the vagus, glossopharyngeal and accessory nerves. Oral growth is characterized by gross brainstem symptoms/early development of intracranial pressure.
The specificity of clinical manifestations is largely determined by the size of the tumor:
- At stage 1, symptoms are manifested by dysfunction of the auditory and trigeminal facial nerves and are accompanied by paresthesia and on the side of the tumor/facial pain of an aching nature, which initially occurs as paroxysms, and later becomes permanent. Along with facial pain paresis of facial muscles , facial asymmetry , loss of taste in the anterior 2/3 of the tongue, impaired salivation , diplopia and convergent strabismus . Stem/hypertensive symptoms are mild or absent.
- At stage 2, disturbances in liquor circulation are noted. Characterized by complete loss of the vestibular/auditory portions of the VIII nerve, complete loss of taste, damage to the V and VI nerves. Brainstem/cerebellar symptoms appear in the form of vertical/horizontal nystagmus , weakening of optonystagmus , and pronounced dysfunctions of the adjacent CNs appear.
- Stage 3 is manifested by tonic nystagmus when looking down, cerebellar disorders intensify, optonystagmus , swallowing and speech disorders appear, and pronounced hypertensive/hydrocephalic symptoms appear.
History of the study
Most experts agree with the statement of the founder of American neurosurgery, H. Cushing, that the neoplasm described in 1777 by Sandifort after autopsy was an acoustic neuroma. Thus, acoustic neuroma was first discovered in the second half of the 18th century.
Sandifort described a dense tumor of the right auditory nerve, fused with the brainstem at the exit of the facial and vestibulocochlear nerves. He also noted its extension into the internal auditory meatus of the temporal bone. Sandifort suggested that it was this formation that was the cause of lifetime deafness.
Harvey Cushing is the founder of American neurosurgery, who made a major contribution to the development of surgery for acoustic neuromas
The pathology of acoustic neuromas was studied by the 19th century French pathologist Cruvelier.
Scottish physiologist and anatomist Charles Bell in 1830 first made a lifetime diagnosis of a tumor of the auditory nerve. The patient had trigeminal neuralgia, loss of taste sensitivity, frequent headaches, and deafness. After 1 year, the patient died. An autopsy revealed a cystic tumor of the cerebellopontine angle extending into the area of the internal auditory canal. An autopsy confirmed Bell's diagnosis was correct.
Walter Dandy - pioneer of acoustic neuroma surgery
The first surgical removal of an acoustic neuroma was performed by the English surgeon Charles Ballance in 1894. The patient lived for at least 12 years after the operation. Ballance removed an encapsulated tumor from the cerebellopontine angle. The clinical description indicates that the tumor was an acoustic neuroma. After surgery, the patient developed a complication that required enucleation of the eye. Damage to the trigeminal and facial nerves was also noted.
H. Cushing made a great contribution to the treatment of acoustic neuromas. His first operation to remove this type of tumor in 1906 ended in the death of the patient. Cushing came to the conclusion that complete removal was impossible using surgical instruments from the early 20th century. In this regard, he began to partially remove the tumor. Initially, mortality after such operations remained extremely high, reaching 40% within 5 years. Cushing's further innovations significantly improved surgical results. In total, Cushing removed 176 acoustic neuromas (in 13 cases he managed to achieve total removal). Postoperative mortality was 7.7%.
In 1917, Cushing's former student W. Dandy demonstrated a successful case of total tumor removal. During 1917-1941, using a unilateral suboccipital approach - removal of the inferolateral part of the occipital bone, Dendy significantly improved the removal technique. In the last 41 cases, the fatality rate was 2.4%. Before the classic works of Cushing and Dandy, the mortality rate for patients who performed operations of this kind was about 75%.
After the introduction of radiosurgery by Swedish neurosurgeon Lars Leksell, it became widely used in the treatment of acoustic neuromas.
Treatment
The treatment algorithm for neuroma is determined by its stage, severity of symptoms, patient’s age and state of health. There are no effective conservative methods for treating acoustic neuromas that would allow controlling the rate of tumor growth and, moreover, achieving its regression. If the tumor is small, has a favorable location (does not compress nearby nerves), and there are contraindications to surgery for various reasons, a wait-and-see approach can be used with dynamic monitoring of the condition of the neuroma by performing CT/MRI. If necessary, drugs of various groups can be prescribed: painkillers, anti-inflammatory, diuretics.
Surgical treatment of acoustic neuroma is aimed at complete (radical) removal of the neuroma while preserving vital brain structures, all functions of the cranial nerves and a minimum of complications. Surgical intervention can be performed through various approaches: translabyrinthine access (through the mastoid process); retrosigmoid access (behind the ear); middle cranial fossa (above the ear).
Each of the surgical approaches has its own indications/contraindications and is selected by the surgeon depending on the size/location of the tumor, the doctor’s preferences and the maximum possibility of preserving nerve function. In the vast majority of cases, the retrosigmoid approach is used in practice.
After the operation, the patient is under observation in the clinic for 7-10 days. Postoperative sutures are removed within 10-15 days. The postoperative period may be accompanied by complications, the most common of which are:
- Postoperative liquorrhea , which is caused by the opening of the mastoid cells (about 10%).
- Meningitis (in 3-4% of cases).
- Postoperative hematomas (hemorrhages) - up to 2% of cases.
- Swelling of the brainstem/cerebellum.
- Paresis of the VI cranial nerve and paresis of other cranial nerves (1-2%).
- Wound infection (1.5%).
After the recovery period, to minimize the risk of tumor regrowth, a course of radiation therapy to eliminate possible remnants of tumor cells.
Radiation therapy. Currently used:
- stereotactic radiosurgery (a method for removing neuromas with a gamma knife);
- fractionated stereotactic radiotherapy (FSRS).
Gamma Knife operations are aimed at preventing further tumor growth and significantly reduce the risk of postoperative complications, and are not inferior in effectiveness to open surgery methods. The biological effects of the method of radiosurgical treatment of neurinomas of the 8th nerve are due to the destruction of tumor DNA and thrombosis of the vessels feeding it. This treatment method is indicated for patients if the size of the neuroma does not exceed 3 cm, as well as in cases of residual/recurrent tumors after surgical removal. Before tumor removal, precise diagnostic imaging of the tumor (three-dimensional reconstruction) is performed to determine the location of the tumor and the nerve structures adjacent to it.
Fractionated stereotactic radiotherapy is carried out in several fractions. Typically, fractionated radiation is used as an adjunct to surgical treatments.
Medicines
—
Procedures and operations
After the operation, the patient must undergo a recovery course, during which he may be prescribed medications to support the nervous system in particular and the body as a whole. During the rehabilitation period, exercises are included aimed at restoring the motor function of the facial muscles.
Acoustic neuromas (Vestibular or acoustic schwannomas)
Acoustic neuromas in 90% of cases originate from the Schwann membrane of the vestibular portion of the VIII cranial nerve and in 10% from the auditory (acoustic). In the medical English-language literature they are referred to as both vestibular and acoustic schwannomas. The clinical manifestations do not differ, so both terms are used. The term acoustic neuroma has become established in Russian-language literature.
There are three main tactics for managing patients with acoustic neuromas:
- dynamic observation
- surgical removal
- stereotactic radiosurgery
In the case of small schwannomas, in the absence of symptoms, or if the patient does not want to be treated, dynamic observation (in the English literature - wait&see or wait&scan tactics, also known as conservative treatment) - periodic monitoring MRIs to identify signs of continued tumor growth. If there are signs of increasing size, removal of the tumor or stereotactic radiation . It should be noted that the option of dynamic observation is traditionally associated specifically with surgical treatment, when the decision to operate should be as justified as possible due to the large number of different risks.
Dynamic observation - monitoring the patient’s condition, monitoring the tumor, as well as other possible concomitant pathological processes in the brain (for example, hydrocephalus). Moreover, during observation, the natural development of the tumor in most cases leads to a gradual decrease and loss of hearing, and the likelihood of surgical or radiosurgery intervention only increases over time. At the same time, stereotactic radiosurgery with Gamma Knife ( SRKhN ), significantly reducing the risk of the need for surgical removal of the tumor, allows one to really fight for the preservation of functional hearing [1]
IMPORTANT! Dynamic observation should not be an end in itself. The vast majority of tumors, once formed, will continue to grow - slowly or quickly, but surely. And in this regard, refusal from surgery or radiosurgery must be clearly motivated, and the patient must be fully aware of what options for the further development of the disease can await him
Clinical example 1 Patient 75 years old. She was examined for unsteadiness when walking and hearing loss. SCT revealed a relatively small tumor in the left cerebellopontine angle, presumably a neuroma. The initial volume of the tumor was 7.8 cc. Surgical treatment was proposed, from which the patient abstained. Subsequently, control MRI was carried out annually, during which a slow increase in the tumor was noted. The patient refrained from surgical treatment. Only after 5 years of follow-up, when the tumor had increased to 10.6 cc, symptoms of trigeminal neuralgia appeared and increased, and the patient once again refused surgery, she was informed about the alternative possibility of performing SRCHN. The patient was warned about the risk of post-radiation edema-swelling of the tumor and the associated risks and gave her consent to undergo SRCHN. 4 months after irradiation, the tumor increased to 11.6 cc, which led to increased trigeminal neuralgia on the left, the development of occlusive hydrocephalus with a decrease in the level of spontaneous activity (the patient is lethargic, adynamic, and needs outside help). Ventriculoperitoneal shunt surgery was performed. Hydrocephalus regressed, the patient's condition returned to normal. Against the background of a gradual (over 2 years) reduction in tumor volume to 9.2 cubic cm (2015), trigeminal neuralgia also significantly regressed. The patient is socialized. Serves itself independently.
Summary: this example clearly demonstrates the futility and unfoundedness of long-term follow-up. Timely implementation of SRCHN with a high degree of probability could avoid deterioration of the patient’s condition and the need for surgical intervention
Rice. 1 Dynamics of changes in VS during its natural growth and after SRHGN
There are still some prejudices and misconceptions regarding the results of using Gamma Knife in the neurosurgical community. In one of the most famous surgical “guidelines,” released in 2009 and devoted to a review of the results of treatment of acoustic schwannomas, the chapter devoted to comparing the results of surgery and radiosurgery begins with a reference to the opinion of the US National Institutes of Health from 1991 (i.e., almost 20 years ago) that the “first line” treatment for most patients is surgery: while radiation is prescribed only to those for whom surgery is contraindicated or they have refused it.
In fact, over the past decade, many studies have been published devoted not only to the results of using the Gamma Knife, but also to comparing them with the results of surgical treatment. And this comparison is not in favor of traditional surgery, even taking into account the current technical capabilities and equipment of neurosurgical departments.
What happens to schwannoma after radiosurgery? 6-18 months after irradiation, acoustic neuromas are characterized by post-radiation pathomorphosis on control tomograms: an area of so-called “central necrosis” that does not accumulate contrast appears inside the tumor, surrounded by a zone of increased accumulation of contrast agent. During this period, the tumor may increase slightly in size. This phenomenon occurs very often during irradiation with schwannomas. This is an expected phenomenon, and most importantly, it is independently reversible. During the first two years after radiosurgery, the presence of such dynamics should not be alarming in any way. If these post-radiation changes drag on for a longer time, it is necessary to clearly differentiate them from the continued growth of the tumor. As 12 years of experience have shown, in 10% of patients, such changes can be observed for more than 2 years, reaching their peak, and then completely regressing at a later date.
Rice. 2. Dynamics of typical changes in acoustic neuroma after irradiation (from left to right: at the time of SRCHN, after 6-24 months, after 2 years)
As can be seen in Figure 2, within 6-24 months, the tumor can significantly increase in size: however, subsequently it decreases significantly, returning to its original size or shrinking. In this series, post-radiation pathomorphosis typical of neuromas was observed in approximately 70% of cases. Moreover, tumor enlargement at this stage occurred only in a third of cases.
The main clinical and radiological patterns of post-radiation pathomorphosis of vestibular schwannomas were described by Pollock in 2006 [2]:
Stereotactic radiosurgery, unlike surgery, does not necessarily aim to remove the tumor. It is important that the tumor does not grow and cause severe, disabling symptoms. No further tumor growth after radiosurgery is the minimum desired result
Is it true that surgical removal of the tumor is a more effective treatment than stereotactic radiosurgery with Gamma Knife?
I will turn to the experience of my work at the Research Institute of Neurosurgery named after. Burdenko:
- From 2005 to 2013, 653 sessions of stereotactic radiosurgery for acoustic neuromas (excluding patients with neurofibromatosis) were performed on 646 patients.
- The ratio of men to women was: 1:2.4.
- The average age of patients is 49 (14-80) years
- The ratio of previously operated patients to primary patients is 1:2. Please note that every third patient who underwent Gamma Knife radiosurgery had previously been operated on and came to us due to the development of tumor recurrence or continued growth of its remnants
- The average follow-up duration is 41.9 months (range 12 months to 8 years)
- Number of relapses – 23
- The average time for relapse to develop is 35.33 months
- Repeated irradiation – 16 patients, with a positive effect in 13 cases
- 10 patients were operated on after SRCHN, incl. 3 after two SRHGN
Thus, the total number of recurrences of acoustic neuromas after Gamma Knife was 3.6%, and in most cases patients underwent re-irradiation - with a positive result, and only in 1.5% of cases surgical removal of the tumor was required
We also treated 180 people whose tumors caused local compression of the adjacent parts of the brainstem and cerebellum, incl. in 59 patients with deformation of the 4th ventricle (stage T4b according to Sami).
The indication for the use of Gamma Knife was the informed choice of the patient in the absence of severe brainstem symptoms, occlusive hydrocephalus, or intracranial hypertension.
- 35 people had previously undergone surgery.
- Audiography before Gamma Knife radiosurgery was performed in 69 patients, 27 of whom were diagnosed with deafness (Gardner-Robertson grade 5).
- The average tumor volume was 6.9 cubic meters. cm (maximum up to 13.8 cc). Maximum diameter (average) – 3.2 cm
- The radiation dose at the tumor edge was 12-13 Gy.
In the case of large vestibular schwannomas: with signs of compression of the brain stem, deformation of the 4th ventricle, i.e. in situations bordering on surgical intervention, the decision to perform SRCHN was made in the absence of gross brainstem disorders, occlusive hydrocephalus and clinical signs of intracranial hypertension. All patients were warned about the possible increase in symptoms against the background of post-radiation changes in the tumor, as well as about the likely need, in this case, for surgical removal of the tumor or ventriculoperitoneal shunting
In 27% of our patients, already at the first control MRI, a decrease in tumor size was noted. At subsequent follow-up MRIs, the number of patients with stable tumor sizes remained approximately the same, and the number of patients with increased and decreased tumor sizes changed significantly. At the last follow-up MRI, 68% of tumors had shrunk after radiation.
Graph 1. Dynamics of changes in tumor volume at different times after SRCHN
Clinical example 2 Patient, 47 years old. intra-extracanal acoustic neuroma. Reduction in tumor volume by 96% just 1 year after irradiation
Clinical example 3. Patient, 45 years old. Small (Sami T3a) acoustic neuroma. Tumor volume regression by 90% in 4.5 years
Clinical example 4 Patient, 48 years old. Major recurrence of acoustic schwannoma 6 years after surgical removal. The tumor volume is 12.5 cc. 4 g after SRCHN there is a decrease in volume by 57%, to 5.4 cc.
Clinical example 5Patient, 32 years old. Recurrence of acoustic neuroma 2 years after surgical removal. Rapid regression of tumor volume (by 88%). The positive effect persists over 10 years of observation
Data published in foreign literature are even more convincing. Neurosurgeons from the University of Pittsburgh [3] published one of the most representative series of patients - 829 patients with vestibular schwannomas and a follow-up period of more than 10 years after SRCHN. Confident control of tumor growth was observed in 98% of patients over 10 or more years of follow-up. In 73% of cases the tumor decreased in size. Size stabilization was observed in 25%. Surgical removal was required in only 0.4% of cases. Surgical treatment of hydrocephalus (shunt surgery) was performed in 0.8% of patients. Thus, our experience and literature data show: radiosurgical treatment is not only not inferior to surgical treatment in terms of controlling tumor growth, but is even superior to it
What functional impairments are possible after Gamma Knife or surgical treatment?
Hearing impairment. Experience shows that hearing remains stable (at the same level as before the use of the Gamma Knife) in 77% of patients. In 75% of patients with functionally preserved hearing, it is also possible to maintain it at the same level.
Violations of facial expressions. Paresis of the facial nerve after Gamma Knife was observed in 8 (1.6%) patients, incl. in 4 (2%) in the group of large schwannomas (T4 according to Sami). At the same time, in 3 patients with paresis of the facial muscles BEFORE radiosurgery, there was a rapid improvement in the function of the facial nerve after irradiation.
Tics (twitching) in the face, of varying severity, incl. and hemifacial spasm, after Gamma Knife, were observed in 17 (3.3%) patients, incl. in 11 (6.1%) in the group of large schwannomas (T4 according to Sami). It is worth noting that in almost all patients, facial nerve paresis and hemifacial spasm regressed completely during drug therapy. The exception was two patients with facial nerve paresis who sought qualified help quite late (after 1 and 3 months), as a result of which the function of the nerve was only partially restored.
Sensitivity disorders on the face. Firstly, this complication is very rare, secondly, it responds very successfully to drug therapy, and thirdly, in most cases of developing decreased sensitivity in a certain area of the face, the quality of life does not change significantly
A decrease in sensitivity on half of the face after Gamma Knife radiosurgery is observed in 3.4% of patients. Only 2% have a persistent character. Worsening of previously existing hypoesthesia is noted in 2.5%
Trigeminal neuralgia after SRCHN occurs in 3.7%, develops within 1-2 years after irradiation, is temporary and regresses with drug therapy
The onset of trigeminal neuropathy averages 6.5 months (range 1 to 11 months).
In patients with deterioration of trigeminal nerve function after irradiation, 67% of the tumors were classified as T4 according to Sami, 33% were classified as T3, i.e. filled the pontocerebellar cistern, in most cases causing local compression of the brainstem.
However, if we compare these complications with surgery, the statistics are clearly not in favor of traditional operations:
In one of the publications devoted to the comparison of the results of surgery and radiosurgery using the example of small (maximum diameter 25 mm) vestibular schwannomas, a significantly more frequent preservation of functional hearing and generally disparate results regarding facial nerve dysfunction are noted[4]
The publication by Chung et al (2010), dedicated to comparing the results of treatment of large (more than 3 cm) vestibular schwannomas, provides meta-analysis data clearly demonstrating the huge percentage of functional complications after surgical removal of tumors. When using the translabyrinthine approach, hearing loss was 100%, and when using the retrosigmoid approach, it was possible to preserve the auditory nerve in only 23.6%. And this is in the largest series (1000 patients) - prof. Sami from Hanover, number 1 neurosurgeon in the treatment of these tumors. The results of preservation of the facial nerve are also not impressive and range from 36 to 86%, i.e. in at least 14% of patients it was not possible to preserve the function of the facial nerve [5]. As for the data of domestic authors, they are discreetly not advertised.
Table 1 Functional outcomes (preservation of the function of the facial and auditory nerves) of microsurgical removal of acoustic neuromas depending on the surgical approach, literature analysis from the article by Chung WY et al (2010)
The following table presents data on stereotactic irradiation of large schwannomas, incl. on GN. In series of GN use, facial nerve function was preserved in 100% of cases, and functional hearing was preserved in 33-100% of patients
Table 2 Functional outcomes (preservation of the function of the facial and auditory nerves) of stereotactic irradiation of acoustic neuromas, literature analysis from the article by Chung WY et al (2010).
From another meta-analysis of the literature presented in the monograph by J. Regis and P. Rocher “Modern treatment of acoustic neuromas” [6] it is also clear that total paresis of the facial nerve in surgical series was observed with a frequency of 12.4 to 43.4%. At the same time, in the GN series, the percentage of gross dysfunction of the FN ranged from 1 to 4.8%.
Table 3 Frequency of development of facial nerve paresis after microsurgical removal of neuromas and after Gamma Knife, literature analysis, from the monograph by Régis J. and Roche P.-H. (2008)
Returning to the already mentioned article by the Pittsburgh group (829 patients and 10 or more years of follow-up), facial nerve function was preserved in 100% of cases, normal trigeminal nerve function in more than 95%: stable hearing level in 70%: and functional hearing in more than 78 % of cases, and improvement in hearing was noted in 1.5% of patients [3]
Thus, Gamma Knife radiosurgery is much safer for the function of the cranial nerves involved in the pathological process than surgical removal of the tumor
Is the risk of developing a radiation-induced tumor after radiosurgery really that bad?
A tumor is considered radioinduced if it meets the following three criteria:
In one of the recent publications [7] devoted to the analysis of the results of treatment of vestibular schwannomas with Gamma Knife with a follow-up period of at least 10 years, one case of the development of a radiation-induced tumor was described among 440 patients. In this regard, the authors analyzed data published in the literature. A total of 15 similar cases were described over 14 years.
Table 4. Radiation-induced malignant tumors in patients with vestibular schwannomas after radiosurgery, literature review from the article by Hasegawa T et al (2013)
At the same time, during the period from 1991 to 2011, Gamma Knife radiosurgery was performed on almost 64 thousand patients around the world
Diagram 1. Number of patients with benign tumors treated with Gamma Knife worldwide (2011, ELEKTA)
Simple calculations show that the probability of developing a radiation-induced tumor after radiosurgery for vestibular schwannoma is approximately 0.025%. At the same time, the average mortality rate after surgical removal of these tumors in recent years is, depending on the clinic, the operating surgeon and many other factors, from 0 to 3% [8]. Thus, the probability of death in the immediate postoperative period is on average approximately 100 times higher than the risk of developing a malignant tumor 3 years or more after Gamma Knife use.
The work of American neurosurgeons on the use of Gamma Knife in pregnant patients indicates that the level of radiation measured in experiment and in practice at the level of critical organs, incl. at the fetal level – extremely low. Therefore, if necessary, it is possible to use Gamma Knife even in pregnant women, in the II-III trimester of pregnancy [9]
English authors analyzed data from 5,000 patients after stereotactic radiosurgery, with a total follow-up of 30,000 patient-years, of which more than 1,200 patients were followed for more than 10 years. So, in this material, only one newly emerging astrocytic tumor was identified, with the expected average probability of the occurrence of such tumors in the population being almost 2.5. This result once again demonstrates that the likelihood of developing a radiation-induced tumor after stereotactic radiosurgery is at least no greater than the risk in any of the people who did not even come close to the Gamma Knife [10]
Is it true that it is much more difficult to operate after previous radiosurgery?
First of all, I would like to note that tumor relapses after Gamma Knife do occur. As I noted earlier, according to our experience and foreign literature, the probability of failure of radiosurgical treatment of acoustic neuromas is estimated at approximately 2-5%, which is completely comparable with foreign data on 5-7% of relapses after surgical removal (domestic data for the last 10 years are missing). In no case in our practice were there any signs of malignancy of schwannomas based on biopsy data. In all cases these were absolutely benign tumors. Probably, the reason for the failure of radiosurgery is the increased resistance of a small number of tumors in the population to ionizing radiation.
In the available foreign literature since 1995, there have been 6 publications [11-16], which discussed the issue of local post-radiation changes after radiosurgery for acoustic neuromas with the Gamma Knife. In total, among the 54 patients analyzed, in 2/3 cases the presence of a local adhesive process was noted, which caused certain difficulties during surgical procedures. However, there is one question that is not answered in any article. Practicing neurosurgeons have no answer either. The question is simple: is there any fundamental difference between adhesions formed after surgery and after radiosurgery? What is the difference between the complexity of surgery after radiation and reoperation after surgery? Judging by the friendly silence, there is not much difference. It is worth thinking: does the occurrence of adhesions after surgery, and the associated difficulties of repeated interventions in cases of tumor recurrence, mean that the tumor does not need to be operated on?
Returning to the issue of post-radiation changes, it should be remembered that the increase in schwannoma after irradiation in the next 1-2 years in the vast majority of cases is a reversible process and in no case is a convincing criterion for continued tumor growth.
Indications for tumor removal, which occur extremely rarely during this period, can only be caused by a clear increase in neurological symptoms: cerebellar ataxia and intracranial hypertension. In this case, surgical intervention should only pursue the goal of reducing the volume of the tumor, and not radical removal, which can lead to severe persistent neurological disorders [17]
Is brain stem swelling possible after Gamma Knife?
For more than 10 years of working with GBV, I have repeatedly heard about the so-called. “swelling of the trunk,” which supposedly necessarily arises as a result of radiosurgery and the danger that lurks in this complication. I heard from doctors, I heard from patients who heard from doctors. But I saw it only once. In this patient, swelling of the trunk appeared 5 months after irradiation, against the background of a temporary increase in tumor size. Even local symptoms did not arise. And it went away on its own, after some time, without any drug support. Our experience shows: using a dose of 12-13 Gy at the edge of the tumor and “correct” planning of radiation is highly effective against the tumor and is safe for the brain stem. In fairness, it must be admitted that at the dawn of the development of radiosurgery, when much larger doses of radiation were used to treat acoustic neuromas (from 20 to 100 Gy) and the side effects were not yet sufficiently studied, cases of the development of brain stem edema were indeed described, but that era ended in the 90s of the XX century.
Is it true that radiosurgery leads to the development of hydrocephalus?
Hydrocephalus with VS may be non-occlusive in nature, i.e. is caused not by compression of the CSF ducts by a large tumor, but by a violation of the relationship between the processes of CSF production by the choroid plexuses of the brain and its reabsorption in the intrathecal space. In our practice and according to the literature, the incidence of hydrocephalus before VS irradiation ranges from 4 to 40%, while the development of hydrocephalus after radiosurgery is about 2%. This is due to an increase in the protein content in the cerebrospinal fluid during VS, and this does not depend in any way on the size of the tumor. This phenomenon either exists or it does not [18]. With the progressive development of hydrocephalus, drug therapy is first carried out, and if this does not help, a simple shunt operation can be performed in any neurosurgical department
Is it true that repeat radiosurgery has a high risk of complications?
Another myth is that repeated radiosurgery has a high risk of complications. However, in reality, the degree of hearing loss (according to the Gardner & Robertson scale), and even more so, facial expression disorders (according to the House & Brekmann scale), actually develop after repeated Gamma Knife extremely rarely [19].
Table 5 Neurological results of repeated Gamma Knife radiosurgery, from the article by Yomo et al (2009) GKS - Gamma Knife radiosurgery, G&R - degree of hearing loss according to the Gardner-Robertson scale, H&B - degree of facial nerve dysfunction according to the House-Brackmann scale
Is it true that radiosurgery is performed only on older people and is not indicated for young patients?
There is no reason to refuse radiosurgery to young patients, because the results of their treatment are no different from the results of elderly patients. Moreover, given the rapid and complete return of able-bodied patients to active life, radiosurgery has a clear advantage over surgical treatment [20].
Is radiosurgery effective for cystic schwannomas?
Over 10 years of work, I have repeatedly heard that cystic schwannomas need to be removed, because... they allegedly do not respond well to radiosurgery. In practice, there has not been a single case of treatment failure for cystic types of schwannomas.
Clinical example 9. Large (volume 13 cc) cystic vestibular schwannoma). After the stage of predictable post-radiation changes in the form of tumor enlargement (after 6 months), the tumor almost halved 1 year after Gamma Knife
Do mobile phones affect the development of acoustic neuromas?
Indeed, the rapid expansion of mobile phone use has raised concerns about possible health risks due to the presence of radio frequency electromagnetic fields from these devices. Therefore, a study of 1105 patients with newly diagnosed acoustic neuroma (vestibular schwannoma) and 2145 controls was conducted in 13 countries using a common protocol.
The study found no increased risk of developing acoustic neuromas with chronic mobile phone use or for users who began using them regularly 10 years or more before the reference date. The researchers speculated that because acoustic neuroma is typically a slow-growing tumor, the interval between the widespread use of mobile phones and the onset of tumors may have been too short to observe an effect, if any.
Regis J. et al (2002) in a study of patients with vestibular schwannomas used objective data and patient responses to a questionnaire to compare the results of radiosurgery (97 consecutive patients treated) and microsurgery (110 patients meeting the selection criteria). The authors concluded that stereotactic radiosurgery was a more effective and less expensive strategy for the management of patients with unilateral vestibular schwannomas up to 3 cm in diameter and was recognized by them as the primary method of choice in the treatment of these tumors [22].
Table 6 Functional outcomes, rehabilitation and social adaptation of patients with VS after surgical treatment and radiosurgery (based on a survey of 207 patients, according to Regis J. et al. (2002)
Berkowitz O et al (2017), having studied in detail the quality of life of 353 patients with vestibular schwannomas an average of 5 years after Gamma Knife, among other facts, noted that 91.1% of patients were satisfied with the functional result and the level of their daily activity, and 96. 8% were willing to recommend this type of treatment to their family and friends if they were diagnosed with such a tumor [23]
CONCLUSIONS:
In the absence of pronounced neurological symptoms affecting the brainstem, SRCHN is a highly effective method of treating VS, incl. significant volume (stage T4 according to Samii)
Dysfunction of the nerves of the cerebellopontine group with SRCHN VS is much less common than with microsurgical removal
The probability of developing radiation-induced tumors after SRCHN VS is extremely small and amounts to no more than 0.03%
Continued growth of vestibular schwannomas after SRCHN occurs in 2-5% of cases. Repeated SRCHN using similar radiation doses along the tumor edge provides a high level of control of tumor growth. The need for surgical removal of the VS after SRCHN occurs only in 1-3% of cases
Consequences and complications
The postoperative period may be accompanied by complications such as liquorrhea , hemorrhage in the surgical site, hemiparesis ; / brainstem edema , meningitis , wound infection, paresis of the 8th cranial and other cranial nerves. Long-term consequences can be manifested by headache , lack of function of the facial nerve, cerebellar disorders, severe disorders of the function of the facial muscles, and bulbar symptoms. And such postoperative complications as oculomotor disorders, swallowing disorders, and paralysis of the facial nerve significantly increase the patient’s risk of disability.
Forecast
The prognosis of neuroma is largely determined by the timeliness of diagnosis/tumor size and the adequacy of treatment. In most cases, with vestibular schwannoma in stages 1-2, with adequate treatment, the prognosis is favorable. With stereotactic radiosurgery in the early stages, cessation of growth/full restoration of the patient’s ability to work is observed in 95%.
In cases of surgical removal of the tumor, there is a high risk of damage to the facial nerve and hearing loss. With stage 3 neuroma, the prognosis is unfavorable with a high risk of death due to compression of vital brain structures by the enlarging tumor.
Pathological anatomy
Main article: Neuroma
Histological image of a neuroma
Macroscopically, neuroma looks like a dense, limited node of round, oval or irregular shape. The surface of the node is uneven, bumpy. Neuroma is covered with a connective tissue capsule. The tumor tissue on the section is pale gray, with areas that have a yellow, rusty tint due to fatty deposits or a brown-brown color (traces of old hemorrhages). The color of the tissue may vary depending on the conditions of the blood supply to the tumor; with venous stagnation, it acquires a bluish tint. In the tumor tissue, there are often cysts of various sizes filled with brown-brown liquid. Cystic degeneration can occur in the entire tumor or part of it. Extensive areas of fibrosis are often observed.
Neuroma consists of spindle-shaped cells with rod-like nuclei. Tumor cells and fibers form “palisade” structures (nuclear palisades, Verocai bodies) with areas consisting of fibers.
Traditionally, two histological types of neuromas are distinguished: Verocai type, or type A, and Anthony type, or type B. This division is conditional and has no practical significance for diagnosis. The Soviet neurosurgeon, academician of the USSR Academy of Medical Sciences B. G. Egorov, when studying acoustic neuromas of the auditory nerve, discovered that their structural diversity does not depend on the initial properties of the tumor tissue, but on destructive and cicatricial processes.
The microscopic structure of the tumor at different stages of growth may be different depending on the intensity of degenerative processes and circulatory disorders. Circulatory disorders are accompanied by the accumulation of hemosiderin and the proliferation of fibrous tissue. All this creates a motley histological picture.
The number of vessels in tumor tissue varies significantly. The peripheral sections are usually surrounded by a rich vascular network: in the central sections their number varies from single to vascular tangles, reminiscent in structure of a cavernous angioma. The walls of the vessels are thin, sometimes formed by a single layer of endothelium, but vessels with sharply thickened hyalinized walls may be encountered.
List of sources
- Rezakova N.V., Gudkova A.A., Pavlova L.V., Luzin R.V., Gaskin V.V. Clinical observation of acoustic neuroma // General Medicine. — 2021. No. 3. pp. 97-99.
- Skobskaya O. E., Kiseleva I.G., Gudkov V.V. Current state of the problem of early diagnosis of vestibular schwannoma // Ukrainian Neurological Journal. 2012.- No. 3. pp. 4-6.
- Gaidar B.V., Khilko V.A., Parfenov V.E., Shcherbuk Yu.A., Martynov B.V., Trufanov G.E., Asaturyan M.A. Tumors of the posterior cranial fossa // Practical neurosurgery / Ed. B.V. Gaidar. - St. Petersburg: Hippocrates, 2002. 648 p.
- Stupak V.V., Pendyurin I.V. Results of surgical treatment of large and giant acoustic neuromas // Modern problems of science and education. – 2021. – No. 5.
- Tastanbekov, M.M. Recurrent vestibular schwannomas: treatment results, features of surgical tactics and technique / M.M. Tastanbekov, T.N. Fadeeva, S.V. Pustovoy et al. // Polenov readings: Materials of the XI All-Russian. scientific-practical conf. - St. Petersburg, 2012. - P. 277.
Reviews of doctors providing the service - Back and spine injuries
In 2000, Andrei Arkadyevich performed spinal surgery on me.
Four days in the clinic and I have been living a full life for 20 years without restrictions on movement and I remember with gratitude Dr. A.A. Khodnevich. God bless him. And in 2000 he could walk no more than 10 meters. Read full review Viktor Alexandrovich
20.05.2020
Low bow to Alexander Semenovich Bronstein and Andrei Arkadyevich Khodnevich. I arrived at CELT on July 2, 2021 with extreme pain that I endured for 10 days. Hernia C6-C-7. I was given two blockades in Ivanovo, about 9 complex IVs, I lost 6 kg in a week and was in a panic, I didn’t see a way out and nothing happened to me... Read full review
Elena Nikolaevna L.
20.10.2019