“Empty sella turcica” syndrome is a condition in which, due to insufficiency of the diaphragm of the sella turcica, the pia mater and tissues located next to it are introduced into the sella cavity and at the same time compress the pituitary gland. A very common disease in the brain area, quickly identified through an MRI study of the sella turcica. Pathologist V. Bush in 1951 was the first to propose the designation “Turkish saddle”, due to the characteristic shape of the sphenoid bone, similar to the well-known part of horse equipment.
What it is?
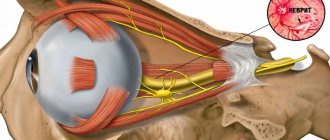
The sella turcica is a term denoting a hollow formation in the body of the sphenoid bone of the human skull in the form of a notch shaped like a saddle, and has a size of 8-12 mm. In the center of the saddle is the pituitary gland, a brain node responsible for the production of hormones that affect growth, reproductive ability and metabolism and is the central organ of the endocrine system and is connected to the hypotholamus. The pituitary gland is separated from the rest of the meninges and the subarachnoid space, filled with cerebrospinal fluid - the septum, and the dura mater - the diaphragm. The hypothalamus is connected to the pituitary gland by a stalk, through an opening in the center of the diaphragm sella, and performs the function of homeostasis and neuroendocrine activity in the body.
In its normal state, the pituitary gland is located in the center of the sella turcica and tends to grow throughout life, occupying the entire space of the pituitary fossa (the part of the sella where the pituitary gland is located). If the integrity of the pituitary gland, the diaphragm sella, is damaged, thinned, deformed, or underdeveloped, the pituitary gland is squeezed by getting the pia mater and cerebrospinal fluid inside the sella and the pituitary fossa. Because of this, the pituitary gland spreads along the lower wall of the sella in the form of a thin layer of tissue, which gave the name to the “empty sella” syndrome. Also, the area of the sella turcica is adjacent to the interweaving of the optic nerves; above it, the optic nerves and tracts partially intersect, which in turn, in the case of distortion of the structure of the pituitary gland, leads to problems associated with vision.
Of course, this deformation of the gland negatively affects its activity, causing hormonal endocrine disorders in the body. Pressure of the contents of the sphenoid bone can also occur as a result of increased intracranial pressure - hypertension, in cases where there is a congenital pathology of the sellar diaphragm and as an additional factor in the acquired syndrome.
Magnetic resonance imaging (MRI) in St. Petersburg
The sella turcica region is a complex complex of closely adjacent anatomical formations located in the brain. Examination of the sella turcica can be part of an MRI of the brain, or an MRI of the sella turcica can be done specifically.
The pituitary gland is an endocrine organ located in the sella turcica. Its mass is only 0.5-1.0 g. The pituitary gland produces a number of hormones, the entry of which into the bloodstream is controlled by releasing factors of the hypothalamus. According to radiographs of the skull, the anterior-posterior dimension of the sella turcica is always larger than the true dimensions of the pituitary gland. On MRI, the normal dimensions of the pituitary gland do not exceed the following: height 8 mm, anteroposterior dimension 12 mm, lateral dimensions 14 mm. Age and gender variations are very significant. An important reference point is the seat diaphragm. Normally it should be slightly concave or flat. The exception is women of puberty and reproductive age. Their diaphragm is slightly elevated and the height of the pituitary gland can be 9-10 mm. During pregnancy, the diaphragm can be elevated, and the height of the pituitary gland can reach 10-12 mm. The size of the pituitary gland also often increases during corticosteroid therapy. There is no clear correlation between the sizes of the pituitary gland and the sella turcica. The internal structure of the pituitary gland is heterogeneous. In the posterior region, in 80-90% of observations, a bright area is visualized. Between the lobes of the pituitary gland, the signal intensity is slightly lower, which is important not to be confused with a Rathke’s pouch cyst.
MRI of the brain. The midline structures of the brain are normal. Sagittal T1-dependent MRI. Designations: Hyp – pituitary gland, I – pituitary infundibulum (pedicle), CHO – optic chiasm, CM – papillary bodies, P – pons, LQ – quadrigeminal plate, OS – sphenoid bone
MRI of the brain. The midline structures of the sella turcica region are normal. Coronal T1-dependent MRI. Designations: Hyp – pituitary gland, V3 – III ventricle, CHO – optic chiasm, ACsup – supraclinoid segment of the ICA, ACint – intracavernous segment of the ICA. The line connecting the supraclinoid and intracavernous segments of the ICA is shown.
Tumors can originate from any tissue and rapidly involve adjacent structures. Therefore, clinical manifestations very often affect both the nervous and endocrine systems. According to the WHO classification, tumors of the central nervous system of the sella turcica region include craniopharyngiomas and some other rare tumors. Pituitary adenomas are tumors of the endocrine system. In the sellar-parasellar region in adults, the first place in frequency is pituitary adenoma, then meningioma and metastasis, aneurysm and craniopharyngioma are less common, neuroma, inflammatory pseudotumor, chordoma, mucocele, Meckel's cavity lymphoma, hypophysitis and Rathke's pouch cyst are very rare. In children, the first place in frequency is occupied by craniopharyngioma, followed by glioma of the optic tract and hypothalamus, rarely neurinoma, arachnoid cyst, germinoma, epidermoid cyst, Rathke's pouch cyst, very rarely hamartoma, teratoma, histiocytosis and meningitis. The MRI technique must necessarily include T1-dependent and T2-dependent sagittal and coronal tomograms. For intrasellar pathology, coronal T1-weighted tomograms with contrast are performed. The cut thickness is selected to be 3 mm or less. In the differential diagnosis of formations in the area of the sella turcica, its localization must be taken into account. If it is only intrasellar, then most likely it is a pituitary adenoma. If it is only suprasellar, it may be a craniopharyngioma, astrocytoma, meningioma, aneurysm, or metastasis. Meningioma, metastases or aneurysm are located parasellar. Almost any tumor can have a combination of intra-, supra-, and parasellar components. Pituitary adenoma accounts for 10 to 15% of all intracranial tumors. The adenoma originates from the anterior lobe, that is, the adenohypophysis. Pituitary adenoma is a benign tumor; malignant forms - adenocarcinomas - are extremely rare. Adenoma occurs at any age, but rarely before 10 years of age and over 60 years of age. Peak frequency occurs at 45-50 years. They are observed equally often in men and women. Based on their size, they are divided into macroadenomas (more than 10 mm) and microadenomas (up to 10 mm). Clinical manifestations consist of endocrine disorders and manifestations of mass effect - headaches, visual disturbances due to compression of the optic chiasm, hypothalamic syndromes. Hydrocephalus is rare. 10% of pituitary adenomas are not clinically manifested; they are called “indentinomas.” These are almost always microadenomas. According to the microscopic type, adenomas are divided into: • acidophilic (eosinophilic), they produce growth hormone or prolactin, • basophilic - produce ACTH, rarely, thyroid-stimulating hormone or gonadotropin • chromophobic - produce prolactin or are hormonally inactive. Prolactin-producing adenomas (30% of cases) cause impotence and decreased libido in men, amenorrhea, galactorrhea, infertility in women, and obesity in all. Growth hormone-producing adenomas (15%) cause gigantism in children and acromegaly in adults. ACTH-producing hormones account for about 10% of cases, the rest are adenomas that excessively produce a combination of hormones. Macroadenomas are usually hormonally active, most often prolactinomas. On MRI of the brain, they extend beyond the normal dimensions of the sella, are usually round in shape, and have a dense capsule. Macroadenoma is usually isointense or slightly hypointense on T1-weighted MRI. T2-dependent MRIs provide poor insight into its size, as it merges with the chiasmatic cistern. It is better to do MRIs that reflect proton density, since on them the adenoma is moderately hyperintense, and the cerebrospinal fluid is hypointense. Inside the adenoma, MRI often shows areas of hemorrhage and cysts, sometimes reaching large sizes; amyloids inside the tumor are described. The tumor is well enhanced on contrast MRI.
MRI of the brain. Pituitary macroadenoma with supra- and retrosellar growth types. Intratumoral cyst. Sagittal and coronal T1-dependent MRI with contrast.
In terms of direction, the growth of a macroadenoma can be: 1. Suprasellar - the upper edge of the tumor is clearly visible against the background of the suprasellar cistern, displacing the optic chiasm. With further growth, it spreads to brain tissue, squeezing the third ventricle and causing occlusive hydrocephalus; 2. Subfrontal - having gone up beyond the saddle, the tumor spreads forward to the base of the frontal lobes; 3. Retrosellar - the tumor goes around the back of the sella and can go down along the slope; 4. Intrasellar - occasionally the tumor “presses through” the bottom of the sella and spreads to the sphenoid sinus. Usually this growth is combined with suprasellar growth; 5. Parasellar - occurs in several variants: a) with displacement of the cavernous sinus and coverage of the internal carotid artery, b) with sinus growth - 6-10% of cases of macroadenomas, c) under the cavernous sinus - extradural growth, d) intradurally between the cavernous sinus and segment of the internal carotid artery passing above the inclined process - supraclinoid intradural growth, e) intradurally between the segment of the internal carotid artery passing above the inclined process and the optic chiasm - supraclinoid intradural growth above the supraclinoid segment of the internal carotid artery. Separately, there are giant adenomas that have all types of growth simultaneously.
MRI of the brain. Pituitary macroadenoma with suprasellar type of growth. Sagittal and coronal T1-dependent MRI, transverse T2-dependent MRI, MRA with partial reconstruction in the axial plane. Compression of the optic chiasm and the third ventricle, A1 segments of the ACA are separated.
MRI of the brain. Pituitary macroadenoma with subfrontal type of growth. Sagittal T1-dependent MRI.
MRI of the brain. Non-secreting macroadenoma. Necrotic center. Extension to the cavernous sinus on the right without invasion of the ICA. T1-dependent MRI with contrast.
The issue of cavernous sinus invasion is extremely important for surgical planning. The horizontal portion of the internal carotid artery (intracavernosal segment) appears as a black circle on coronal MRIs of the brain, reflecting fast-moving blood. Displacement of the ICA on MRI does not indicate sinus invasion. It is believed that the probability of invasion is very high when covering more than 70% of the diameter of the ICA or when crossing the line connecting the mid-diameters of the intracavernous and supraclinoid segments of the ICA on coronal sections. The spread of the tumor to the bones of the skull base is clearly visible by a decrease in the signal from the cancellous substance on T1-dependent MRI.
MRI of the brain. Pituitary macroadenoma with infra- and parasellar growth types. VCA germination. Coronal T1-dependent MRI.
MRI of the brain. Pituitary macroadenoma with parasellar growth on the right. VCA germination. Coronal T1-dependent MRI.
It is highly advisable to do an MRI to monitor the response of prolactinoma to treatment with inhibitors of its secretion (bromocriptine, Destinex). With effective treatment, the size of the tumor on MRI decreases, and its structure undergoes cystic degeneration. Macroadenomas can undergo apoplexy - an acute disruption of the blood supply to the pituitary gland with necrosis or hemorrhage. The incidence of apoplexy is about 10%. It is observed at the age of 37-57 years, 2 times more often in men. Clinically, apoplexy is an acute condition in the form of headache, vomiting and visual disturbances. On MRI, areas of heterogeneity appear in the adenoma in the form of hemorrhage or cysts. A special option is necrosis of the neurohypophysis in pregnant women - Sheehan syndrome. MRI of the sella turcica makes it possible to identify and differentiate this condition.
MRI of the brain. Pituitary macroadenoma with suprasellar type of growth. Hemorrhage into the tumor. Coronal T1-dependent MRI.
Microadenomas do not have a capsule or clear boundaries. Some of them do not manifest themselves clinically, others are hormonally active, excessively producing prolactin or ACTH. The standard MRI protocol for a suspected pituitary microadenoma consists of sagittal and coronal T1-dependent MRIs performed before contrast, slice thickness -2 mm, optionally transverse T2-dependent MRIs are performed. After contrast, it is necessary to do coronal T1-dependent MRI, possibly with fat suppression (FatSat), optionally a dynamic study or 3D SPGR type MRI (turboFLASH).
MRI of the brain. Typical pituitary microadenoma. Part of the standard examination protocol: T1-dependent, T2-dependent and T1-dependent contrast-enhanced coronal MRI.
It is sometimes possible to increase the sensitivity of the method using the dynamic MRI contrast method. A 0.1 mM/kg contrast agent is injected quickly as a bolus and tomography begins immediately. MRI coronal slices and an accelerated technique, such as 3D turbo SE, are optimal. Contrast enhancement on MRI of normal pituitary tissue begins in the posterior lobe and quickly spreads to the anterior lobe. As a rule, MRI contrasts normal tissues faster than tumor tissues. The optimal time for detecting microadenoma is about 90 s. from injection. Interpretation of images must be cautious, as there is a danger of mistaking the intermediate part between the pituitary lobes or a Rathke's pouch cyst for an adenoma.
MRI of the brain. Pituitary microadenoma (arrow). T1-dependent tomogram with conventional and bolus contrast.
Indirect MRI signs allow one to suspect a microadenoma: elevation of the sella diaphragm, obliquity of the fundus and/or stalk of the pituitary gland. The last two symptoms look at coronal T1-dependent MRI. Iris asymmetry of up to 1.5 mm is allowed on coronal MRIs. Direct signs, that is, the tumor itself, if it is less than 5 mm, may not be found. If a microadenoma is nevertheless detected, it is usually hypointense on T1-dependent and hyperintense on T2-dependent MRI. About 20-30% of microadenomas are hyperintense on T1-dependent MRI and may be hypointense on T2-dependent MRI. Microadenoma on MRI may contain cystic and hemorrhagic components. The absence of MRI findings in patients with endocrine disorders raises the question of further tactics. It is advisable to resort to MRI with contrast. Microadenomas after contrasting appear to be more hypointense than the surrounding normal pituitary tissues, which are enhanced. In rare cases, after gadolinium administration, a microadenoma enhances more than surrounding tissue on MRI, and is more likely to be an ACTH-producing microadenoma. Craniopharyngioma arises from epithelial cells remaining from the involuted craniopharyngeal canal (Rathke's duct). It is a benign, slow-growing, extra-axial tumor. In general, its frequency is slightly more than 3-5% of intracranial tumors and 6-10% of tumors in children. In children, craniopharyngioma is the most common tumor not related to the neuroepithelial series; in them it accounts for more than half of all tumors of the sella region. In them, it is usually of the adamantine type and has a significant cystic component. In adults, craniopharyngiomas are often of the papillary type and consist of a solid component without cysts and without calcifications.
According to MRI, suprasellar location is observed in 75% of cases, supra-intrasellar - 21% and isolated intrasellar - 4%. Based on the nature of growth visible on MRI of the brain, the following types are distinguished: Type A – the tumor is almost entirely intrasellar, PCoA and the A1 segment of the ACA are not displaced; Type B - the tumor grows anterior to the optic chiasm and displaces it posteriorly. PCoA and A1 segment of the ACA are elevated, the OA is not displaced Type C - the tumor grows posterior to the optic chiasm and displaces it anteriorly. Obstruction of the third ventricle and hydrocephalus are observed. PCoA and A1 segment of PMA. Craniopharyngioma is asymptomatic for a long time, then manifests itself with nausea, vomiting, headaches and visual impairment - bitemporal hemianopsia, nipple swelling and optic nerve atrophy, as well as endocrine disorders - growth retardation, obesity, hypothyroidism, occasionally diabetes mellitus and early maturation. On MRI, the craniopharyngioma is clearly demarcated, surrounded by slight edema, and is often hypointense on T1-dependent and hyperintense on T2-dependent MRI. The adamantine tumor subtype has a significant cystic component on MRI, and the solid component often contains calcifications, giving it a “spotty” appearance. The cystic component of the tumor on MRI often contains an admixture of protein, blood and cholesterol. The papillary subtype on MRI consists entirely of a solid component and does not contain calcifications. Craniopharyngioma enhances well on contrast-enhanced MRI, usually more peripherally.
MRI of the brain. Craniopharyngioma (arrow). Adamantine type. T1-dependent sagittal MRI before and after contrast.
Astrocytoma originates from the optic nerves, chiasm, and tracts. It accounts for about 5% of primary CNS tumors in children. Some cases are manifestations of neurofibromatosis type I. Astrocytoma can also originate from hypothalamic tissue and spread secondarily to the chiasm and tract. Histologically, the tumor belongs to the pilocytic subtype and does not disseminate through the subarachnoid spaces. Astrocytomas of this localization grow very slowly, usually expansively. If the tumor spreads upward, it can block the foramina of Monroe, causing occlusive hydrocephalus. On MRI of the brain, the astrocytoma is hypointense on T1-dependent MRI and hyperintense on T2-dependent MRI. The internal structure of the tumor on MRI is homogeneous. MRI with contrast does not always enhance, but if contrast does occur, it is usually of a peripheral type. Germinoma , although much more common in the pineal gland, can also be localized in the hypothalamus, the pituitary infundibulum and the anterior segment of the third ventricle. Often, suprasellar localization is combined with a tumor in the pineal gland region, that is, a primary multiple tumor. Typically, germinoma is diagnosed in patients under the age of 20 years, most often female. Clinically manifested by diabetes insipidus and visual field impairment. MRI of the brain reveals an iso- or hypointense formation on T1-weighted MRI and slightly hyperintense on T2-weighted MRI; it is clear that the tumor is localized in the midline, homogeneous in structure, has an infiltrative growth pattern, and is well enhanced on contrast-enhanced MRI. The tumor grows quickly and can disseminate throughout the subarachnoid spaces and ventricles. Meningioma of supra- and parasellar localization occurs in adults. It originates from the diaphragm sellae, the membranes covering the anterior oblique process, the tubercle sellae and the cavernous sinus. If the meningioma spreads towards the optic chiasm, it causes compression and visual disturbances. The tumor can grow into the cavernous sinus, compressing the nerves and internal carotid artery located in it. When growing forward along the base of the skull (subfrontal type of growth), the tumor sometimes extends into the orbit and sphenoid sinus. When spreading backward, meningioma can “slide” along the clivus. MRI signs of meningioma in this location do not differ from those in any other area. Relatively low signal on T2-dependent MRI and good contrast enhancement and dural tails help differentiate it from other suprasellar tumors. The absence of an intrasellar component on MRI distinguishes it from a pituitary adenoma.
MRI of the brain. Suprasellar meningioma. "Dural tails" (arrows). Sagittal T1-dependent MRI with contrast.
Metastases mainly enter through the hematogenous route. They make up less than 1% of pituitary tumors. The primary localization of the tumor is the mammary gland (the pituitary gland occupies 6-8% of all areas of metastasis) and the lung. Only 7% of pituitary metastases manifest clinically, usually as diabetes insipidus. On MRI of the brain, the hallmark of metastasis is thickening of the pituitary stalk, disappearance of the high signal behind the posterior lobe of the pituitary gland, and invasion of the cavernous sinus is also typical. Metastasis is usually isointense on T1- and T2-dependent MRI.
In addition to the pituitary gland itself, metastasis can be located in any of the structures of the sellar-parasellar region. It is very difficult to distinguish it from other tumors, but metastasis can be suspected by bone destruction, sellar sclerosis and multiplicity. Schwannoma (neurinoma) is a rare parasellar tumor. Arises from the III and V pairs of cranial nerves, less often the IV and VI pairs. The clinical picture of neuromas consists of pain and atrophy of the muscles they innervate. With MRI, a neuroma can be localized anywhere along the course of nerves and their branches. Differential MRI diagnosis of tumors should also be carried out with non-tumor diseases: with intrasellar localization - with Rathke's pouch cyst and granulomatous inflammation, as well as the "empty" sella syndrome; supra- and parasellar localization - with epidermoid and arachnoid cysts, teratoma, hypothalamic hamartoma, aneurysm, sarcoidosis and histiocytosis. Granulomatous inflammation of the pituitary tissue (hypophysitis) is a rare condition caused by tuberculosis, sarcoidosis, syphilis, mycotic lesions, or rupture of Rathke's pouch cyst. Clinically, hypophysitis is manifested by headaches and increased prolactin levels. On MRI, hypophysitis resembles a pituitary adenoma. The increase in the size of the pituitary gland disappears after steroid therapy and specific antibiotic therapy. Empty sella syndrome is a congenital (primary empty sella) or acquired (secondary empty sella resulting from surgery or radiation therapy) defect of the sella diaphragm. The diaphragm is an outgrowth of the dura mater, extending from the dorsum to the tubercle and laterally between the dural walls of the cavernous sinuses. In its central part there is a hole where the hypothalamic stalk enters. In the presence of a defect in the diaphragm, the chiasmal cistern prolapses into the saddle (“hernia” of the cistern) until the bone formations are pressed through. The syndrome is present in 8-35% of the population. This is usually an asymptomatic condition. But sometimes it can manifest as obesity (80% of the symptomatic “empty” sella), headaches (80%), memory impairment, nausea, cramps, rhinorrhea, visual disturbances (10%) and hypopituitarism. Hormonal deficiency is associated with secondary atrophy of pituitary tissue. The peak frequency of detection of the syndrome occurs at the age of 40-49 years. Radiographs show an increase in the size of the saddle, its closed configuration is preserved, the bottom is thinned, but symmetrical. On CT, the contents of the sella often have low density, but due to the phenomenon of beam enhancement, the density may be soft tissue, which makes differential diagnosis with adenoma difficult. An MRI reveals cerebrospinal fluid contents inside the sella turcica, and the pituitary gland is “flattened” along the bottom. The pituitary stalk is usually displaced posteriorly and, unlike Rathke's pouch cyst, crosses the sella towards the remnants of the pituitary gland. In the differential diagnosis of an “empty” sella, it should be remembered that after 50 years, involution of the pituitary gland is observed. On MRI in such patients, the chiasmal cistern may prolapse slightly into the sella.
MRI of the brain. "Empty" sella turcica. Sagittal T1-dependent MRI.
MRI of the sella turcica in St. Petersburg can be done with equal success in open MRI and closed “tunnel” machines. In MRI centers in St. Petersburg, open machines do not use dynamic contrast.
Leave feedback.
MRI in St. Petersburg USA
Types of pathology
Statistically, every tenth person is diagnosed with the “empty sella” syndrome. It is most common in women aged 35 to 55 years and is directly related to obesity (75%) and the activities of the female body - pregnancy and menopause (abortion is believed to be an additional factor).
Scientists highlight the need to separate the types of syndrome:
- Primary or idiopathic - as a consequence of congenital deficiency, asymptomatic, discovered mainly by chance on MRI during examination for another disease.
- Secondary - after radiation and surgical treatment or infections - is accompanied by unpleasant symptoms.
The tendency to the syndrome can be hereditary or a consequence of the influence of various factors.
How to prepare for an MRI of the sella turcica?
Eating a light meal 30 minutes before contrast will help prevent nausea, dizziness, drooling, and metallic taste.
In most medical centers, diagnostics are carried out by appointment. When planning a native study, no special measures are required: it is enough to rest well before the procedure and choose clothes without metal parts.
Women during lactation are advised to stock up on milk for 2-3 feedings; within 24 hours, the contrast agent will completely leave the body.
Before an MRI scan, it is better to refrain from drinking alcohol and smoking.
The clinic will require the following documents:
- passport;
- doctor's referral indicating the preliminary diagnosis, area and type of examination (with or without contrast);
- extracts from the medical record, hospital, conclusion of the oncology consultation;
- results of previously performed MRI, radiography.
Causes of “empty sella turcica”
Medicine has identified several causes for the occurrence and diagnosis of the syndrome.
- Congenital deformation of the diaphragm and hereditary predisposition;
- Multiple pregnancies, miscarriages and menopause;
- Necrosis of the pituitary gland as a result of ischemia, also as a consequence of cardiovascular diseases;
- Long-term use of hormonal drugs and contraceptives;
- Past infectious diseases;
- Mechanical impacts and traumatic brain injuries;
- Brain tumors;
- Consequences of cancer treatment;
- Obesity, antibiotic abuse, etc.
Diagnostics
A study of the sella turcica area involves neuroimaging of parts of the brain that are inside or nearby - the diaphragm, pituitary gland, hypothalamus, pituitary stalk, optic nerves. Instrumental diagnostics in MRI format gives an idea of the location and morphological structure of the listed brain structures. Other methods:
- Ophthalmological examination (in the presence of chiasmal syndrome). Includes visometry, ophthalmoscopy, perimetry. Differential diagnosis for empty sella syndrome is carried out in relation to glaucoma.
- Angiography (study of the circulatory system in the adjacent area).
- CT scan.
To confirm endocrine disorders, a blood test is performed and the concentration of pituitary hormones is determined. If necessary, additional x-rays of the bone structures of the skull are performed.
Symptoms of an “empty sella turcica”
Often the clinical manifestation of the syndrome is asymptomatic or with periodic increases in symptoms, as well as combinations of endocrinological and neurological manifestations.
Symptoms related to the neurological part of the disease manifestations.
- Neuropathological and visual disorders - blurred vision, black spots in the eyes and increased tearing;
- Headaches, with a lack of localization and increasing intensity, do not depend on the time of day or the person’s state of sleep or wakefulness;
- Surges in blood pressure, often accompanied by fainting, shortness of breath or chills, diarrhea, chest pain;
- Increase in body temperature to minor borderline levels;
- Abdominal pain, convulsive reactions.
Endocrine signals from the body indicating abnormalities in pituitary function.
- Obesity – 75% of patients are overweight;
- Sexual dysfunction in men and enlarged mammary glands;
- Overactivity of the thyroid gland and as a consequence: increased sweating, tremors of the hands and eyelids, emotional instability, faster heartbeat;
- Insufficient (hypo) functioning of the thyroid gland: fatigue, constipation, swelling, dry skin;
- Menstrual irregularities, manifestations of infertility;
- Decreased adrenal function - Itsenko-Cushing syndrome - is characterized by increased hair growth, mental disorders, and pigmentation.
Of course, all of the above is relevant to many other diseases, so to diagnose the syndrome, a targeted examination is necessary.
Diagnosis of the syndrome
The difficulty of identifying the disease requires a combination of several types of diagnostics.
- Preliminary, contact diagnosis by a doctor - after a comprehensive analysis of the patient’s complaints and comparing them with the medical history - referral for a substantive examination;
- Study of clinical blood parameters - blood test for thyroid hormones (hormones produced by the thyroid gland);
- Hardware research. Several electronic diagnostic devices can be used here - sometimes they are required in combination. MRI provides the most accurate picture of the state of brain disorders. An MRI shows how deformed the pituitary gland is, its subsidence and the degree of displacement. Next, CT (computed tomography) diagnostics will allow the specialist to accurately determine the size of the gland and possible shifts in location. There is also an X-ray - a lateral projection of the skull and a targeted examination of the desired area, but it cannot give a clear picture and can only be used as a primary option, before a more clear diagnosis.
Often, an “empty sella turcica” is identified in the process of diagnosing other, associated or unrelated diseases.
Which doctor should I go to?
If a person notices strange unrelated headaches, unreasonably rapid weight gain or uncontrollable hypertension, then of course, it is necessary to contact a therapist - a doctor who, based on complaints and obvious prerequisites, will already refer you for examination and consultation: to a neurologist - for identifying or ruling out neurological problems; to an ophthalmologist - in order to exclude, in case of anything, deterioration related directly to the visual channels or to other diseases; to an endocrinologist to evaluate the indications of laboratory hormonal tests. And then, based on the collected material, the doctor will be able to accurately diagnose and develop an effective plan for monitoring the condition and offer treatment options for the patient.
Functions
The functions of the brain structures located in the sella turcica in the head are associated with the work of the endocrine system and the visual apparatus, which, in the case of the development of pathologies, predetermines such disorders as a decrease in the acuity and quality of vision. The hypothalamic-pituitary system provides neurohumoral regulation in the body. Underdevelopment of bone structures, damage to areas of the brain, volumetric intracranial processes localized in this area often provoke diseases.
Treatment
Treatment of “empty sella turcica” is mainly symptomatic. Doctors must first accurately diagnose the syndrome; if it turns out that it is a congenital pathology, then treatment is not required. The patient is registered with a specialized medical institution to control and monitor the disease.
If the disease is acquired (secondary), then treatment is prescribed by the doctor based on the presence of the patient’s underlying problems.
- High blood pressure - vascular therapy and observation by a therapist and cardiologist;
- Vision problems - monitoring and auxiliary treatment by an ophthalmologist;
- Poor tests - a strong increase or decrease in thyroid hormonal parameters - replacement maintenance therapy with drugs from the field of endocrine diseases;
- Frequent migraines, weak immunity - your therapist will also recommend additional medications to eliminate these symptoms.
Contacting a surgeon is not often required and only in extreme cases when there is no other choice. Although surgery is not uncommon, it is part of the usual treatment and is prescribed if it is believed that it will relieve the patient of the problem. But, nevertheless, often this is a last resort to eliminate serious problems:
- Removal of brain tumors of various etiologies;
- Sagging of the optic nerves and tracts into the opening of the diaphragm - there is a danger of complete loss of vision;
- Leakage of cerebrospinal fluid through the hole in the sellar diaphragm and leakage of CSF from the nose.
Traditional treatment is ineffective, but it does work as a general strengthening of the body and saturating it with vitamins. Also, you should stop taking hormonal drugs, develop a weight loss plan and eliminate dietary excesses.
Anatomical structure
The anatomy of the skull of newborns suggests a cup-shaped sella turcica with a wide entrance hole, as can be seen in the photographs. The upper part of the back of the saddle is represented by a cartilaginous structure, and therefore is not visible in the photographs. Cartilage tissue becomes ossified by the baby's first year of life. The subarachnoid (under the arachnoid membrane) space is separated from the area of the sella turcica by a hard shell.
The area of adjacent dura mater is known as the diaphragm. The pituitary gland is connected to the hypothalamus through a stalk. The leg passes through the diaphragm. During puberty, the saddle acquires an individual shape, which, identical to fingerprints, allows one to identify an individual from the bones of the skull using intravital craniograms (X-rays).
Typically, a similar identification method is used in forensic medical examination. The formation in the skull is limited from above by the diaphragm, and from the sides by the cavernous sinuses. Above the area of the sella turcica, a depression in the skull bone, is the chiasma of the optic nerves. By the age of 3, the formation in the skull becomes round.
During puberty (sexual development), the shape changes from round to oblong. The normal dimensions of the sella turcica in adults in the sagittal direction are about 9-15 mm. The dimensions of the sella turcica in the vertical direction are about 7-13 mm. If after puberty the saddle remains round, this indicates infantilism (developmental immaturity).
4. Treatment of meningioma of the tubercle of the sella turcica
As mentioned above, this type of tumor is extremely difficult to surgically remove due to its deep localization and proximity to vital brain centers. The most effective is radical surgical removal
neoplasms using transcranial access - with craniotomy. It should be noted that such an extensive operation is, of course, quite traumatic for the patient, but in most cases it is unfortunately impossible to completely remove the tumor using more gentle surgical methods.
With a favorable location of the meningioma and its small size, in some cases it is possible to perform the operation using a more gentle transsphenoidal (transnasal) approach
- through the nasal passages.