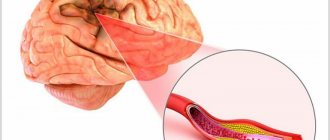
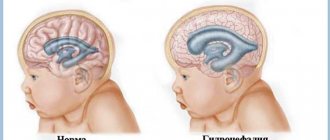
Cerebral ischemia, to put it simply, is an insufficient supply of blood to the child’s brain. This can lead to disability and even cause death. In most cases, hypoxic-ischemic encephalopathy is diagnosed, and not strictly ischemia in recently born babies. Hypoxic-ischemic encephalopathy means that the cells do not receive the necessary doses of oxygen, which is why pathological processes begin in the brain.
- Causes
- Pathogenesis (essence of pathology)
- Risk factors for pathology
- Degrees
- Signs of severe ischemia
- Cerebral ischemia in prematurity and in full-term infants
- Hypoxia results
- Diagnostics
- Treatment
- Prevention of ischemia
Signs and symptoms of cerebral ischemia in newborns
The disease manifests itself with obvious symptoms that attract attention.
- The child is easily excitable, cries for no reason, sleeps poorly, shudders, and has tremors.
- Muscle tone is reduced, the baby moves little, has difficulty sucking and swallowing.
- The fontanel is enlarged, intracranial pressure is increased due to the fact that fluid accumulates in the brain.
- Convulsions, twitching of the limbs and head, as well as comatose states with loss of coordination of movements and consciousness occur.
- The newborn's skin takes on a marbled hue.
- The functioning of the gastrointestinal tract is disrupted - bloating, constipation, and diarrhea are observed.
Clinical manifestations
Symptoms of the disease vary significantly at different stages. And if the first degree does not require placing the child in a hospital and the signs disappear within a few days after the disease, then the second and third require close attention from doctors and, if necessary, resuscitation measures.
Symptoms of CI 1st degree
The pathological condition is characterized by mild damage to the tissues of the central nervous system, followed by the development of oxygen starvation. The cause of the disease is usually difficult childbirth with injuries and asphyxia. Symptoms are mild. Therefore, it is difficult to determine the presence of the disease. Because of this, adverse consequences may develop.
The most typical manifestations are:
- Severe headaches and migraines. They manifest themselves in the form of restless behavior, sleep problems and tension. The baby will cry often.
- Increased muscle tone is possible. The muscular system is constantly tense. Dense when touched with fingers. The degree of hypertonicity is determined by a specialist, since muscle tension is normal for infants.
- Increased response to tendon reflexes. Information is collected by tapping the knee joint with your fingers. In this case, the limb bends very quickly.
How does cerebral ischemia occur?
In 70% of cases, ischemia occurs in the fetus in the womb and is associated with the formation of a blood clot in one of the vessels supplying the brain, or with insufficient development of the vessel. Most often, the disease is diagnosed in premature babies whose vascular system is not yet fully formed.
As a result, an insufficient amount of blood enters the vital organ, and with it, oxygen. Delay in providing medical care leads to damage to larger areas of the brain, cerebral hemorrhage and other serious consequences.
Premature birth is a leading cause of infant mortality and a significant factor in the loss of human potential of surviving children during later life. According to foreign authors, several million children with very low body weight (VLBW) are born every year around the world [1]. In the United States, 90% of the 65,000 newborn VLBW infants survive the neonatal period due to great advances in intensive care, but 5–10% of these children are later diagnosed with cerebral palsy [2].
The introduction of modern medical technologies in the last decade has been marked by a decrease in perinatal and infant mortality. At the same time, an increase in the survival rate of children with VLBW and extremely low body weight (ELBW) at birth entails an increase in morbidity and the formation of early disability [3]. Among the causes of childhood disability, pathology of the nervous system ranks first, and the contribution of perinatal lesions reaches 60–80% of all neurological diseases [4]. In Russia, annually no more than 2.5-5% of those examined as disabled since childhood are recognized as able to work, compared to 50% abroad [5].
Among the factors that adversely affect the antenatal period, disruption of the uteroplacental circulation, which can be caused by both extragenital and somatic pathology of the mother, is of great importance [6]. Disturbances in the uteroplacental blood flow, in turn, lead to the development of hypoxia, which is the central link in the pathogenesis of antenatal damage to the fetus, and primarily to the central nervous system (CNS). The works of many authors have established a whole complex of physiological adaptive reactions of the fetus to unfavorable developmental conditions, in particular to hypoxia [7-10]. However, there is insufficient information on the biochemical status of the nervous tissue of the fetus and newborn in this pathology. Of great interest in this regard is the study of glucose metabolism, the characteristics of free radical oxidation and glutamate metabolism, the processes of necrosis and apoptosis.
In utero, the fetus is in a state of hypoxia, but this environment is physiological for it, moreover, in the early stages of the embryonic period it is necessary for normal cell differentiation. Fetal oxygenation depends on oxygen partial pressure gradients between maternal and placental blood, fetal blood and fetal tissue. It is known that in the first weeks after conception in the embryonic period, the level of partial pressure of oxygen (pO2) is extremely low and amounts to about 18-20 mm Hg. Presumably, this is necessary to protect the embryo, which is very sensitive to the damaging effects of reactive oxygen species [11]. Hypoxia in the embryonic period causes angiogenesis and is a prerequisite for maintaining pluripotency of stem cells [12]. It is noteworthy that in the first trimester of pregnancy, embryonic stem cells develop at a pO2 level of about 10-15 mm Hg, while in the endometrium, pO2 is about 25 mm Hg. Stem cells demonstrate more efficient growth and differentiation at low oxygen pressures of 10-15 mmHg. [13]. Prolonged hypoxia will stimulate angiogenesis through transcriptional and post-transcriptional regulation of growth factors: vascular endothelial growth factor, erythropoietin, placental growth factor and angiopoietin1 [14].
The main regulator of adaptive cell responses to hypoxia is hypoxia-inducible factor 1 (HIF-1), a heterodimeric transcription factor including subunits (HIF-1α and IGF-1β). IGF-1α stabilizes when the oxygen concentration is below a certain critical threshold, thus accumulating in a hypoxic environment. IGF-1β is present in the cell nucleus, and under hypoxic conditions it dimerizes with IGF-1α, improving oxygen delivery to the tissue [15, 16]. At the 14-16th week of pregnancy, pO2 rises to stable values of 45-50 mmHg. and remains so until the end of pregnancy. In late pregnancy, the rate of cell proliferation and differentiation decreases [17, 18]. Lipid peroxidation processes are present from the very beginning of pregnancy, which contributes to the normal development of the fetus. At the end of the first trimester, physiological oxidative stress causes regression of the villi that were formed throughout the surface of the chorionic sac to form the final discoid placenta [19]. The postnatal increase in oxygen concentration causes a surge in the formation of its reactive species, with the expression of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase dynamically increasing during the last weeks of pregnancy. Similarly, the availability of the most important non-enzymatic antioxidants increases: glutathione, heme oxygenase, vitamins C and E, β-carotenes, etc. [20]. The premature infant is at greater risk of free radical damage [21]. The use of high concentrations of oxygen during neonatal resuscitation is thought to cause hyperoxemia. At the same time, a significant correlation was found between oxidized glutathione (GSSG), pO2 and the activity of enzymes in the glutathione redox cycle [22, 23]. Many diseases associated with prematurity, such as retinopathy, bronchopulmonary dysplasia, and intraventricular hemorrhage, are associated with free radical damage as a result of the immaturity of the antioxidant system of preterm infants [24].
Nervous tissue is most vulnerable when exposed to hypoxia. Hypoxia leads to disruption of the exchange of oxygen and carbon dioxide, which in turn causes metabolic disorders and hemodynamic disorders [25]. The following mechanisms underlying cerebral damage during hypoxia-ischemia are known: local disturbances in the metabolism of high-energy compounds, excessive lipid peroxidation and disturbance of Na+/K+-ATPase activity, extracellular accumulation of K+ and intracellular accumulation of Ca2+, intracellular acidosis, disturbance of neurotransmitter metabolism [26 ]. The main links of hypoxic-ischemic stress are presented by P. Marro [27].
- Lack of oxygen. A deficiency of oxygen as an electron acceptor in tissues leads to disruption of electron transport in the Krebs cycle and respiratory chain, replenishment of energy by increasing cerebral blood flow and anaerobic metabolism [28].
— Glutamate-calcium cascade. An increase in glutamate concentration activates N-methyl-D-aspartate (NMDA) receptors, which is accompanied by an increase in intracellular Ca2+ [29]. Disturbances in mitochondria and endoplasmic reticulum can lead to further accumulation of intracellular Ca2+. An increase in Ca2+ concentration inside the cell promotes the formation of free radicals, which in turn causes lipid peroxidation of the cellular and intracellular membranes. Along with this, the accumulation of intracellular Ca2+ is naturally accompanied by an increase in its concentration in the cell nucleus. Excess intranuclear Ca2+ is a factor in the activation of protoapoptotic genes, which trigger genetically programmed cell death - apoptosis [30].
— The role of free radicals. Hypoxia-ischemia causes inadequate saturation of mitochondrial cytochrome oxidase, disruption of electron transport in mitochondria, which leads to an increase in the concentration of superoxide anion and the entry of free radicals from mitochondria into the cytoplasm [31]. An increase in intracellular Ca2+ concentration activates NO synthetase, cyclooxygenase and lipoxygenase, which promotes the formation of free radicals. Their excess leads to additional release of excitatory amino acids and activation of NMDA receptors [32].
- Inflammatory factors. The effect of hypoxia-ischemia on microglia promotes the synthesis of cytokines, interleukin-1β (IL-1β), tumor necrosis factor α (TNFα) [33]. IL-1β activity is accompanied by the production of specific proteases and the development of apoptosis. Excessive production of TNFα has a direct toxic effect and causes vascular infiltration with the release of cytotoxic factors, reactive oxygen species and cytokines [34].
— The role of nitric oxide (NO). NO synthetase is found in endothelial cells, astrocytes, and neurons. There are 3 isoforms of NO synthetase: neuronal (regulates synaptogenesis and remodeling and depends on Ca2+); endothelial (regulates vascular tone, especially vasodilation, and depends on Ca2+); inducible (present in macrophages and astrocytes, induced by cytokines, independent of Ca2+) [35]. Activation of NMDA receptors causes the production of neuronal NO synthetase, which promotes the formation of nitric oxide (NO.) radical and damage to neuronal DNA [36].
- Apoptosis. The processes described above develop in the first minutes of acute hypoxia, after which the apoptosis mechanism is activated [37]. Hypoxia, through a number of pathogenesis links, promotes the accumulation of intracellular Ca2+, activation of endonucleases, and damage to gene expression. This leads to disinhibition of the phagocytic activity of glial cells and neurons, which phagocytose the damaged neuron, causing a decrease in its size and sequestration [38].
The most significant loss of nervous tissue cells develops 2-6-48 hours after birth, due to pathological oxidative stress. Under such conditions, in the first hours and days of life after birth, newborns who have suffered hypoxia develop a pronounced imbalance in the cerebral circulation regulation system, which aggravates the course of the ischemic process [39].
A feature of premature infants is the immaturity of the antioxidant system, since a physiological increase in antioxidant capacity occurs at the end of pregnancy, which is why they are more susceptible to oxidative stress, especially when their condition requires respiratory therapy [40]. In this regard, there is a need to study oxidative stress in these children, in particular by measuring lipid peroxidation products and components of the antioxidant system.
Antioxidants are known to have anti-inflammatory activity, and the glutathione system is considered a critical factor in the development of inflammation and immune responses [41, 42]. This is confirmed by changes in the levels of cytokines, acute phase proteins and glutathione during inflammation [43, 44]. The glutathione system includes its forms, a number of enzymes for its synthesis and catabolism, and transport mechanisms. All these components make an important contribution to changes in glutathione status [45].
When studying the classification of cerebral lesions in newborns, it should be noted that the most popular among neonatologists is the classification of hypoxic encephalopathy according to H. Sarnat and M. Sarnat [46]. It combines clinical signs of cerebral ischemia and electroencephalography (EEG) results. This classification evaluates the main indicators of a newborn: level of consciousness, neuromuscular status, reflexes, autonomic function, presence of seizures, EEG. Depending on the severity of cerebral dysfunction, stage I, II or III of encephalopathy is established. Canadian neonatologists modified the Sarnat classification, adding thermoregulation disorders and excluding EEG and some other indicators [47]. Neonatologists in Great Britain use the classification of hypoxic cerebral disorders by L. Dubowitz et al.[48]. In the International Classification of Diseases, 11th revision (ICD-11), hypoxic-ischemic brain lesions in newborns belong to the group of “neurological disorders characteristic of the perinatal and neonatal periods” [49]. The main difference from the classification of the 10th revision is that in ICD-11 this group is supplemented with diseases that were not previously identified separately. For example, perinatal arterial stroke and neonatal cerebral sinovenous thrombosis. At the same time, congenital hydrocephalus was excluded from the proposed classification. The diagnosis of newborn asphyxia was placed in the “group of other disorders arising in the perinatal period.” At the same time, newborn asphyxia with an Apgar score of 0-3 points and newborn asphyxia with an Apgar score of 4-6 points were separately identified. As for cerebral injuries of a hypoxic-hemorrhagic nature, they were classified into the group of “hemorrhagic and hematological disorders in the fetus and newborn.” At the same time, the classification of intraventricular hemorrhages has changed somewhat. In ICD-11, it is customary to distinguish 4 degrees of intraventricular hemorrhage, while in ICD-10 the 3rd and 4th degrees were combined.
Symptoms of severe central nervous system damage may not appear immediately after birth, but may occur several hours later. However, clinical symptoms do not always reflect the true severity of the disease. In this regard, intravital assessment of changes that occur in the cells of nervous tissue in the early neonatal period is of particular relevance. Ultrasound examination (ultrasound) allows us to identify structural changes in the brain of newborns due to perinatal hypoxic damage to the central nervous system. Analysis of ultrasound and pathomorphological data indicates that the nature of ischemic brain damage depends not only on the severity of perinatal hypoxia, but also on the maturity of the child [50]. In full-term newborns, cerebral ischemia is accompanied by the occurrence of selective neuronal necrosis, subcortical and multicystic encephalomalacia, and cerebral infarctions [51]. Severe perinatal hypoxia in premature infants 34–37 weeks of gestation, as a rule, leads to the development of periventricular leukomalacia. In connection with the nursing of extremely premature infants with ELBW, ultrasound has become more likely to detect such forms of ischemic damage as diffuse leukomalacia and periventricular hemorrhagic infarction. It should be noted that in the first 24-48 hours of the debut of neonatal arterial ischemic stroke, this method does not have sufficient sensitivity and specificity, since the focus of ischemic damage begins to appear only on the 2-3rd day from the onset of ischemia, which is associated with the course of pathohistological processes. The severity of the inflammatory reaction, the intensity of necrosis and apoptosis reach their peak 48–72 hours after circulatory disturbance [52]. This leads to a change in the echogenicity of the damaged brain parenchyma. Further assessment of the evolution of the ischemic focus is not inferior in information content to magnetic resonance imaging (MRI) [53, 54]. The use of Doppler ultrasound helps to increase the sensitivity of ultrasound in the early stages of cerebral damage. Duplex scanning allows you to objectively assess the hemodynamic characteristics of cerebral vessels. The value of Doppler ultrasound technique in the acute period of the disease is the identification of the vasodilation phase, the earliest sign of ischemic damage, which occurs within 30 minutes after the development of a vascular accident and persists for the first 5-6 days [55]. Vasodilation develops in response to the action of various metabolites and increases the supply of glucose and oxygen to ischemic tissue. Its characteristic features are an increase in blood flow velocity and a decrease in peripheral resistance indices in the damaged vascular system [56, 57]. MRI has become the most informative method for diagnosing perinatal brain damage. The presence of various pulse sequences provides high sensitivity and specificity, even in the early stages of the development of a vascular accident [58, 59]. MRI with diffusion-weighted images and the construction of maps of the measured diffusion coefficient (MCD) makes it possible to detect an ischemic lesion within 30 minutes from the moment of its occurrence. ICD serves as a quantitative characteristic of diffusion in tissue and reflects the presence of intracellular edema [60]. The presented diagnostic methods are necessary to identify, determine the localization, severity of brain damage and prognosis. Their disadvantages are the short diagnostic time interval and the limited possibility of repeated examination [61]. Over the past 20 years, the diagnostic value of biomarkers has been studied to predict the outcome of cerebral injury in newborns [62, 63]. Considering the pathophysiological changes that occur as a result of damage to brain tissue, neuroproteins, calcium-binding protein, vasoactive substances, markers of oxidative stress, inflammatory mediators, etc. have been studied in detail [64-67]. However, despite the promise of studying biomarkers, there is no data on their practical use in medicine.
Treatment of hypoxic brain lesions is the subject of heated debate and extreme opinions, ranging from complete denial of the need for treatment with neurotropic drugs to aggressive polypharmacy [26]. According to modern views, hypoxic-ischemic encephalopathy occurs during asphyxia, usually in the structure of multiple organ disorders, therefore the main principle of therapy is to remove the child from asphyxia and maintain vital functions [68]. Specific treatment for hypoxic-ischemic encephalopathy is therapy for cerebral edema and neuroprotection, which primarily involves controlling the volume of cerebrospinal fluid (CSF), cerebral perfusion and the volume of brain matter. Cerebral perfusion depends on arterial inflow, venous outflow and the metabolic rate of nervous tissue. At this stage, the adequacy of artificial ventilation and the effectiveness of hypothermia are of great importance. It should be noted that hypothermia is carried out in newborns with a gestational age of at least 36 weeks if at least one of the signs set out in the special criteria is present [69]. Control of fluid volume in the CSF pathways is achieved by inhibiting the production of CSF and improving its outflow. Controlling brain volume involves enhancing active transport and stabilizing neuronal membranes. It should be noted that at the moment the use of drugs in neonatology is limited due to the high risk of side effects and lack of evidence base. However, the search for such drugs continues to this day. In particular, over the past two decades there have been significant changes in the understanding of the role of erythropoietin as a neuroprotector. Erythropoietin has been shown to be involved in neurogenesis and angiogenesis during embryonic development and is activated after brain injury [70], and also has a cytoprotective effect on endothelial, glial cells and neurons [71]. The neuroprotective role of erythropoietin was first identified in several in vitro
and
in vivo
[72]. At the same time, it was found that erythropoietin has anti-apoptotic [73], antioxidant [74] and anti-inflammatory effects [75]. In addition, it attenuates the effects of inflammation by reducing reactive astrocytosis and suppressing microglial activation, and reduces the number of immune cells at the site of inflammation [76]. The absence of endogenous erythropoietin is known to increase ischemic brain damage and worsen neuronal survival [77]. The established protective effects of erythropoietin during ischemia and reperfusion have prompted the use of recombinant erythropoietin in premature infants with cerebral ischemia, intraventricular hemorrhage, and periventricular leukomalacia [78]. Thus, one study assessed the effectiveness and safety of erythropoietin in neonatal hypoxic-ischemic encephalopathy. This study included 167 newborns with moderate to severe cerebral damage. All children were divided into two groups. Children of the first group received standard therapy for hypoxic-ischemic encephalopathy, and children of the second group received erythropoietin at a dosage of 300 and 500 U/kg to standard therapy. The drug was administered in the first 48 hours every other day for 2 weeks. It turned out that mortality and disability were 19.2% more common in the group of children who did not receive erythropoietin [79].
Thus, the problem of ischemic cerebral damage in premature newborns is very relevant. First of all, this is confirmed by the high incidence of this pathology and the high risk of death and disability in children. The question of complex diagnosis of cerebral disorders in premature infants remains open, because there is no single algorithm that combines data from the clinical picture, instrumental and laboratory research methods. Of great interest is the search for predictors of unfavorable outcome in order to optimize treatment approaches.
The authors declare
no conflict of interest.
The authors declare no conflicts of interest.
Information about authors
Anuriev A.M. - https://orcid.org/0000-0002-6724-5067; e-mail
Gorbachev V.I. — https :// orcid . org /0000-0001-6278-9332
How to quote:
Anuriev A.M., Gorbachev V.I. Hypoxic-ischemic brain lesions in premature newborns. Journal of Neurology and Psychiatry. S.S. Korsakov.
2019;119(8 issue 2):63-69. https://doi.org/10.17116/jnevro201911908263
Corresponding author:
— Anuriev Alexey Mikhailovich — e-mail
Causes
In the vast majority of cases, the causes of cerebral ischemia in newborns are various disorders of gestation in recent weeks, as well as non-standard situations during childbirth.
- Detachment of the placenta or disruption of blood flow in it.
- Compression of the umbilical cord, suffocation of the fetus.
- Congenital heart defects.
- Circulatory problems.
- Intrauterine hypoxia.
- Infection during childbirth.
- Openness of the ductus arteriosus.
- Acute placental insufficiency.
Etiology
The causes of heart problems in children are conventionally divided into large subgroups. Let's look at each one separately.
Mother's side
If during pregnancy a woman has suffered from rubella, systemic lupus erythematosus, syphilis, toxoplasmosis, if she has diabetes mellitus or other chronic diseases, then all this is reflected in the fetus.
The list of approved drugs for pregnant women is strictly limited; most antibiotics, warfarin and other drugs (they are called teratogenic) are too toxic for the fetus. Taking them increases the risk of spontaneous abortion (miscarriage) or various abnormalities in the development of the child.
Drinking alcohol, smoking and using drugs during pregnancy also leads to congenital pathologies in children.
Genetic disorders
Most often these are chromosomal abnormalities. But genetic predisposition and mutations are not considered the only cause of cardiac pathologies. In 90% of cases, the cause of heart problems is multifactorial.
Bad habits
We have already talked about how the state of the mother’s body affects children. But the risk factors for developing heart problems in children are similar to those in adults:
- Smoking;
- Alcohol;
- Sedentary lifestyle;
- Narcotic substances;
- Stress;
- Infections;
- High cholesterol levels.
This list is more relevant for teenagers 14-17 years old than for kids. However, we must understand that nicotine and ethanol metabolism products destroy the myocardium and vascular wall in a child in the same way as in an adult. “Pampering” behind garages can result in a heart attack at age 30.
Statistics show that over the last decade the percentage of children with excess body weight has increased, which also negatively affects the heart. Myocardial infarction, due to obesity and other risk factors, has become “younger” and is already occurring in girls and boys aged 20-30 years, which was considered casuistry back in the 2000s.
Risk factors
Various vascular and neurological pathologies, problems with blood pressure (especially hereditary) in the mother should alert the doctor who is managing the pregnancy. Also, risk factors for cerebral ischemia in a child are:
- mother's age is more than 35 years;
- endocrine diseases;
- premature, prolonged labor;
- multiple pregnancy;
- late toxicosis;
- failure of the mother to follow a healthy lifestyle;
- exacerbation of chronic or acute diseases in the mother during pregnancy.
Why does it occur
Hypoxic manifestations are observed if a woman is pregnant or during childbirth. Oxygen starvation is provoked by the following circumstances:
- The presence of infections in the body of a pregnant woman, dysfunction of the cardiovascular and endocrine systems, acute respiratory infections.
- When drinking alcoholic beverages, smoking.
- With late toxicosis, reduced volume of amniotic fluid, pregnancy of more than one fetus, late labor.
- Placental and umbilical cord pathology.
- If the baby is premature.
- In case of a difficult birth, which consists in the fact that the baby is entwined with the umbilical cord, the birth continues longer than expected due to a large fetus, trauma during childbirth and other problems of labor.
- The child’s mother is over 34 years old or under 18.
All these factors disrupt the blood flow between the placenta and the uterus, which is manifested by a hypoxic state.
Diagnostics
The disease is usually diagnosed within the first few hours.
The presence of pathology is indicated by deviations in checking reflexes and a general blood test . Typically, the analysis shows an increased level of carbon dioxide in the body.
If obvious symptoms of a serious illness are detected magnetic resonance imaging , as well as electroencephalography , which reveals hidden convulsions and other abnormalities in the functioning of the brain.
Diagnostic criteria
The initial diagnosis consists of examining the child and assessing his condition using the Apgar scale.
Determination of pH of venous blood to detect acidosis
Examination of a newborn includes the following studies:
- Blood test (reduced oxygen concentration).
- Neurosanography.
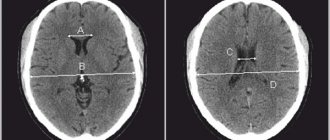
- CT scan.
- Magnetic resonance imaging.
- Doppler encephalogram.
Cerebral ischemia grade 2
A dangerous form of the disease. It is characterized by:
- severe apnea (stopping breathing during sleep);
- decreased grasping and sucking reflexes;
- weak muscle tone;
- increase in head shape due to fluid accumulation;
- lack of coordination;
- loss of consciousness;
- changes in skin color.
Most often, grade 2 ischemia manifests itself in the first day of a newborn’s life, and symptoms can be observed for 2-4 weeks. At this time, the child is carefully monitored by doctors, and he undergoes a course of therapy. If necessary, surgery is performed to remove the blood clot.
Prevention of ischemia
It is necessary to talk about pregnancy in advance. Preparations begin half a year, or at most 3 months before conception. And not only the expectant mother, but also the father of the child must prepare. During gestation, a woman should undergo all examinations on time, especially in the first trimester. Tests are taken and ultrasound diagnostics are performed.
Before and during pregnancy, a woman is examined for infections. As is known, after conception, latent infections can worsen and manifest themselves. Mom needs to give up smoking, alcohol, drugs and other habits that are harmful to her and the baby. Smoking has a particularly negative effect on the process of oxygen supply to the fetus. If the doctor discovers pregnancy complications, it is important for the woman to go to the hospital on time.
Cerebral ischemia grade 3
The most severe form, in which:
- the baby has no reflexes;
- the child falls into a coma;
- heart rhythm is disrupted;
- blood pressure rises sharply;
- there are problems with independent breathing;
- strabismus is observed.
An experienced doctor can already determine the presence of signs and symptoms of grade 3 cerebral ischemia in the first 5 minutes of a newborn’s life. In this case, the child is sent to intensive care and, if necessary, connected to a ventilator.
Hypoxia results
A lack of oxygen to the brain can have the following results:
- Severe cerebral ischemia
This condition is fatal in a quarter to half of cases. The babies do not survive even a few days. Another scenario is pneumonia or another infection that kills the child a little later. Most of those who do not die are diagnosed with autism, cerebral palsy or dementia. And only 10% of survivors experience no consequences.
- Moderate ischemia
According to statistics, severe long-term consequences occur in 30 to 50% of children.
- Mild ischemia
The outcome in most cases is favorable, no disability is noted.
Treatment of cerebral ischemia in newborns
The goal of treatment is to restore normal blood circulation in the brain tissue, prevent pathological changes and eliminate the consequences of ischemia. For stage 1 disease, treatment usually involves prescribing massage to improve blood circulation.
For diseases of the 2nd and 3rd degrees, drug therapy and surgery are used to remove a blood clot in the vessel and restore the structure of the vascular bed. In difficult cases, the baby undergoes a rehabilitation course of intensive therapy.
Features of pathology in full-term and premature infants
The nature of damage to the anatomical structures of the brain during hypoxia or asphyxia depends on the gestational age. Thus, at the birth of a premature baby, there is a risk of developing periventricular leukomalacia. With this disease, damage to the white matter occurs with the further formation of foci of necrosis. Children born before 31 obstetric weeks are susceptible to pathology.
In full-term infants, the gray matter is predominantly affected. The degree of complications depends on the extent of the disorder and the anatomical location of the neurons. In the case of severe, acutely developing hypoxia, pathological changes are observed in the area of the brain stem, which coordinates the work of the heart and breathing. Deviations can cause dire consequences and cause irreparable harm to the baby’s health.
Prognosis and consequences of cerebral ischemia
It is much more effective to eliminate ischemia itself after the birth of a baby than to treat its complications. Among the consequences of cerebral ischemia of the 2nd degree:
- sleep disorders;
- headache;
- irritability;
- isolation;
- physical inactivity.
- Stage 3 disease has severe consequences:
- cerebral palsy;
- attention deficit disorder;
- mental retardation;
- Graefe's symptom, etc.
If all actions to eliminate ischemia were carried out by doctors in a timely manner, then the symptoms disappear during the rehabilitation period, which usually lasts 6-12 months.
Symptoms
A child who has heart problems may complain of:
- Chest pain (in the area of the heart, possibly radiating to the arm, hypochondrium, or armpit);
- Shortness of breath;
- Rapid heartbeat or noticeable pauses;
- Interruptions in heart function;
- Dizziness;
- Darkening of the eyes and fainting;
- Paleness or cyanosis (bluish coloration of the skin and mucous membranes);
- Acceleration of the pulse or its difference in the hands;
- Poor appetite and short stature;
- Frequent respiratory infections;
- Swelling and pulsation of neck vessels;
- High or low blood pressure;
- Chest deformation, etc.
Symptoms usually begin or worsen after exercise. At first, the child usually does not attach any importance to this, then his endurance begins to suffer, and his condition worsens, which becomes the reason for contacting a cardiologist.
Self-diagnosis
To independently diagnose the state of the cardiovascular system, functional tests are carried out: Martinet-Kushelevsky, Kotov-Deshin, Rufier, with a 15-second run at the fastest pace, and others. You can familiarize yourself with the sampling methodology in the relevant articles on the Internet.
These are simple tests to roughly assess the adaptation of the heart and blood vessels to stress. In schools and universities, they are carried out in special medical physical education groups to monitor the well-being and fitness of students.
Complications
If you have cardiac problems, medical supervision is always necessary. In the youngest patients, the pathology may disappear on its own, but this is a rare occurrence. Teenagers during puberty are often worried about arrhythmia or tachycardia (increased heart rate) - this is the price to pay for hormonal changes; in most cases, everything returns to normal on its own.
Despite the presence of happy outcomes, if a child has heart complaints, you should urgently consult a cardiologist.
Otherwise, complications may arise:
- Secondary arterial hypertension (in the systemic and/or pulmonary circulation);
- Destruction of valves and adaptive changes of the heart (dilatation or hypertrophy;
- Poor circulation (other organs suffer, ischemia occurs - insufficient oxygen supply to the tissues);
- Heart attack;
- Pneumonia;
- Heart failure.
Death is a common result of heart pathologies, especially with congenital defects.
Online consultation with a pediatrician
consultation cost: 500 rubles
Online consultation
During the consultation, you will be able to voice your problem, the doctor will clarify the situation, interpret the tests, answer your questions and give the necessary recommendations.