Encephalopathy is a complex of neurological and mental disorders associated with focal damage to the brain. Depending on the etiology, several forms of this disease are distinguished: dyscirculatory, which is caused by circulatory pathology and is more common in old age, traumatic, which usually occurs in athletes after repeated traumatic brain injuries, hypoxic, etc. Encephalopathy of toxic origin develops due to direct damaging effects on the brain of various toxic substances. Pathology can occur at any age, including in children, but the clinical course and prognosis largely depend on the general state of health and timely initiation of treatment. Is this pathology treatable? Doctors at Leto clinics are convinced that with rapid detoxification and elimination of the toxin, the chance of complete restoration of the functioning of the central nervous system (CNS) is very high.
Toxic encephalopathy of the brain: what kind of disease, main causes
The toxicogenic effect of most neurotropic poisons is based on their negative impact directly on neuronal membranes and the mechanisms of nerve impulse transmission. Violations of energy and plastic metabolism play an important role in the pathogenesis of the disease. In addition, some toxins inhibit the hematopoietic and circulatory systems, breathing, which also causes toxic hypoxic encephalopathy. Due to the peculiarities of the anatomical structure, cells localized in the cerebral cortex, cerebellum and hippocampus are especially sensitive to the influence of pathological factors, which causes the clinical manifestations of the disease.
Major neurotoxins
The main causes of toxic encephalopathy include:
alcohol and volatile solvents: destroy cell membranes, receptors, cause changes (irreversible with long-term exposure) in energy metabolism;- psychoactive substances (opiates, cannabinoids, amphetamines, cocaine, ephedrine, hallucinogens, some drugs, etc.): pathologically affect the reuptake system and the production of norepinephrine, dopamine and serotonin
- heavy metals (mercury, lead, manganese, arsenic, thallium, etc.): have a direct destructive effect on the structures of the central nervous system and also affect the functioning of the peripheral nervous system.
Accordingly, the risk of toxic brain encephalopathy is especially high among people working in hazardous industries. These are, for example, chemical, construction, metallurgy, and agriculture, where insecticides and pesticides are used. The disease is often diagnosed in alcoholism and drug addiction. The pathology is less common among artists; those who use neurotoxic paint solvents are at risk.
Causes of development of alcoholic encephalopathies
The cause of the development of this disease in the vast majority of cases is long-term consumption of large doses of alcohol. Typically, the onset of symptoms is preceded by binge drinking that lasts for weeks or months, or habitual daily drinking for many years. The risk of developing the disease increases when consuming technical fluids and alcohol substitutes. Sometimes, due to the individual characteristics of the patient’s body, the disease develops in the absence of alcoholism, against the background of regular consumption of small doses of alcohol or rare alcoholic excesses.
The disease is based on metabolic disorders characteristic of alcoholism. The leading role is played by the lack of vitamins (primarily group B). With constant consumption of alcohol, the body's need for vitamin B1 increases, and its level decreases. This is due to an irregular, monotonous diet, lack of appetite during binges, deterioration in the absorption of vitamin B1 in the intestines and impaired liver function. The consequence of B1 deficiency is metabolic disorders in the brain. The problem is aggravated by a lack of vitamin P and B6. Due to a deficiency of these vitamins, the functions of the digestive system are further impaired, and the capillaries in the brain become more permeable, which can lead to cerebral edema.
Depending on the rate of development, predominant symptoms, course characteristics and outcome options, two groups of alcoholic encephalopathies are distinguished: acute and chronic. Acute encephalopathies include hemorrhagic polyencephalitis (Haye-Wernicke syndrome), mitigated acute encephalopathy and fulminant encephalopathy. The history of patients suffering from chronic encephalopathies usually reveals severe alcoholic psychoses or acute encephalopathies. There are two forms of chronic alcoholic encephalopathy: Korsakov's psychosis (alcoholic paralysis, polyneuritic psychosis) and alcoholic pseudoparalysis.
Clinical manifestations of toxic encephalopathy
The signs of the disease and the course of the disease largely depend on the neurotoxin that caused this condition. Depending on the severity and duration of symptoms, the disease can be acute or chronic. But even the acute form rarely goes away without any consequences. Residual effects persist for a long time (and in some cases throughout life). Typically this is:
- regular headaches with dizziness;
- general weakness, increased fatigue;
- irritability;
- sleep disturbances (difficulty falling asleep, frequent awakenings, nightmares);
- autonomic dysfunction syndrome, which is manifested by increased sweating, tremor of the fingers of outstretched arms, and decreased muscle tone;
- cognitive disorders: deterioration of memory and attention; often the patient experiences difficulties even with reading fiction and watching TV shows and films.
After acute toxic encephalopathy, hypothalamic crises may periodically occur, accompanied by:
- increased headache;
- rise in blood pressure;
- pain syndrome in the chest area;
- chills with trembling;
- attacks of anger and irritability.
Symptoms of the acute form of the disease
This type of illness is typical for poisoning with heavy metals and volatile solvents; it is relatively rare in alcoholism and drug addiction, usually against the background of an already existing deficiency or disturbances in thiamine metabolism.
Clinical manifestations of the pathology include:
- oculomotor disorders (eye paralysis, strabismus, decreased response to light stimuli);
- astasia and abasia (loss of the ability to move without support and assistance);
- generalized convulsive syndrome;
- amnesia, and often the patient replaces events that he does not remember with fictitious ones;
- oral automatisms;
- repeated vomiting;
- severe headache;
- increase or decrease in body temperature;
- tachycardia;
- acute psychosis.
Without timely medical care, there is a high risk of death due to cerebral edema, stroke, and acute respiratory failure.
Symptoms of chronic toxic encephalopathy
With a prolonged course, there are 5 stages of the disease:
- Prodromal. Signs of the disease can only be visible to the patient’s loved ones. Pay attention to lack of concentration, absent-mindedness and forgetfulness.
- First. Apathy develops, coordination of movements decreases, mild tremors are possible, sleep disturbances, loss of appetite, and weight loss are typical. Various emotional disorders are noted: feelings of internal tension and anxiety, fear.
- Second. Personality and behavioral disorders come to the fore, which many take to be the initial symptoms of dementia.
- Third. Speech disorders, severe disorientation, and a decrease in almost all reflexes are characteristic.
- Fourth. Toxic encephalopathy of the most severe degree: coma develops with loss of all neurological functions.
Very often the disease is complicated by concomitant acute and chronic somatic pathologies. Thus, in addition to neurological symptoms, manifestations from the gastrointestinal tract and cardiovascular system are often noted. As a rule, the immune system suffers greatly, which leads to a decrease in resistance to various bacterial, viral and fungal infections. And in such cases, the negative influence of waste products of pathogenic microflora “joins” the action of the neurotropic poison - infectious-toxic encephalopathy develops.
Perinatal hypoxic encephalopathy
Risk factors. Etiology and pathogenesis of hypoxic encephalopathy
To recognize the clinical signs of perinatal hypoxia, it is necessary to take into account risk factors predisposing to its development [8]: • Borderline maternal age (younger than 20 and older than 35 years) • Premature placental abruption • Placenta previa • Preeclampsia • Premature or late labor • Staining of amniotic fluid with meconium • Bradycardia, fetal tachycardia, muffled fetal heart sounds • Multiple pregnancy • Prolonged anhydrous interval • Maternal diabetes • Any illness of the mother during pregnancy • Taking drugs potentially dangerous to the fetus by the mother Among the causes of disturbances in pulmonary ventilation and blood oxygenation, peripheral and central hypoxia are distinguished. In peripheral hypoxia, pathology of the respiratory tract or alveolar blood flow is involved; in central hypoxia, the basis is a dysfunction of the respiratory center.
Etiology of hypoxia
| Peripheral | Central |
| 1. Respiratory | 1. Low blood pressure |
| distress syndrome | at the mother's |
| 2. Aspiration of amniotic fluid | 2. Maternal anemia |
| water | |
| 3. Pneumothorax | 3. Arterial hypertension |
| (with birth trauma) | |
| 4. Bronchopulmonary | 4. Placental |
| dysplasia | failure |
| 5. Congenital anomalies (Pierre-Robin syndrome) | 5. Brain malformations |
Hypoxia leads to disruption of oxidative processes, the development of acidosis, a decrease in the energy balance of the cell, an excess of neurotransmitters, and disruption of the metabolism of glia and neurons. Acidosis increases the permeability of the vascular wall with the development of intercellular edema and disruption of cerebral hemodynamics. Under hypoxic conditions, lipid peroxidation is disrupted with the accumulation of aggressive free radicals and hydroperoxides, which have a destructive effect on neuronal membranes. Disturbances of cerebral hemodynamics of an ischemic-hemorrhagic nature are a consequence of severe brain hypoxia. In the antenatal period, the main etiological factor of hypoxia is placental insufficiency [2,9]. Trophic insufficiency occurs with impaired absorption and assimilation of nutrients through the placenta, deficiency of oxygen and carbon dioxide transport, which is manifested by fetal growth retardation syndrome, intrauterine hypotrophy, immaturity of the lungs and surfactant. It has been established that a decrease in uteroplacental blood flow serves as an objective indicator of hypoxic brain damage [4]. Surfactant deficiency and respiratory anoxia are the main pathogenetic factors of cerebral hypoxia in premature infants and newborns from diabetic mothers. The pathogenesis of neonatal surfactant deficiency is secondary fetal hyperinsulinism, which develops in response to maternal glycemia. Insulin inhibits the synthesis of lecithin, the main element of surfactant, the deficiency of which prevents the alveoli from sticking together, which leads to impaired ventilation of the lungs. Thus, compensation for diabetes in pregnant women is the prevention of respiratory distress syndrome in newborns and hypoxic encephalopathy [15]. In the neonatal period, the cause of cerebral hypoxia and ischemia can be a pronounced intrapulmonary shunt. Hypoxic cardiopathy of newborns and adrenal insufficiency play a significant role in the pathogenesis of brain hypoxia. Hypoglycemia and deficiency of glycogen reserves are considered as factors causing increased vulnerability of brain tissue to hypoxia in newborns, especially premature infants with low body weight.
Morphology of hypoxic encephalopathy
The brain normally absorbs a fifth of the oxygen entering the body. In young children, the brain's share of oxygen use is almost half, which ensures a high level of oxidative metabolism. Depending on the duration of hypoxia, changes develop in the brain from local edema to necrosis with hemorrhagic impregnation. A number of studies have shown that there is varying sensitivity of brain structures to hypoxia, which depends on the characteristics of metabolism and blood supply. The most sensitive to hypoxia are Sommer's area of the ammon's horn and the periventricular region of the adjacent blood supply between the anterior, middle and posterior cerebral arteries. When hypoxia and ischemia are combined, foci of necrosis occur in the cortex, thalamus optic, striatum, and cerebellum. The following stages of morphological hypoxic changes in the brain are distinguished: stage I - edematous-hemorrhagic, stage II - encephalic gliosis, stage III - leukomalacia (necrosis), stage IV - leukomalacia with hemorrhage. The first two stages of acute hypoxia are curable, it is possible to restore the metabolism of neurons and glia, stages III and IV lead to irreversible death of neurons. With antenatal hypoxia, neuronal degeneration, glial proliferation, sclerosis phenomena, and cystic cavities at the sites of small foci of necrosis are observed.
Hypoxic encephalopathy clinic
In the clinical picture of hypoxic encephalopathy, three periods are distinguished: acute (1st month of the child’s life), recovery (from 1st month to 1 year, and in premature immature children up to 2 years) and outcome [12]. In the acute period, according to the degree of severity, mild forms of damage to the nervous system are distinguished, reflecting transient disturbances of hemolytic fluid dynamics; moderate form with edematous-hemorrhagic changes, gliosis, single leukomalacia; severe form, characteristic of generalized cerebral edema, multiple leukomalacia and hemorrhages. The Apgar scale is used to determine the severity and severity of cerebral circulatory disorders. In the acute period, 5 clinical syndromes are distinguished: increased neuro-reflex excitability, convulsive, hypertensive-hydrocephalic, depression syndrome, comatose. Usually there is a combination of several syndromes. A feature of the acute period is the dominance of general cerebral disorders without pronounced local symptoms. In mild forms of brain damage (Apgar score 6 - 7 points), a syndrome of increased neuro-reflex excitability
.
The main manifestations of the syndrome are increased spontaneous motor activity, restless shallow sleep, prolongation of the period of active wakefulness, difficulty falling asleep, frequent unmotivated crying, revitalization of unconditioned innate reflexes, muscle dystonia, increased knee reflexes, tremor of the limbs and chin. In premature infants, the syndrome of neuro-reflex excitability in 94% of cases is a clinical sign of a decrease in the threshold of convulsive readiness, which is confirmed by electroencephalography (EEG) data [12]. Patients who, according to EEG data, have a decrease in the seizure threshold should be considered at risk for seizure syndrome. The moderate form of hypoxic encephalopathy (estimated on the Apgar scale 4 - 6 points) is manifested by hypertensive-hydrocephalic syndrome and depression syndrome
.
Hypertensive-hydrocephalic syndrome
is characterized by an increase in head size by 1 - 2 cm compared to the norm (or chest circumference), opening of the sagittal suture of more than 0.5 cm, enlargement and bulging of the large fontanelle.
The typical shape of the head is brachiocephalic with enlarged frontal tubercles or dolichocephalic - with the occiput hanging posteriorly. Graefe's symptom, the "setting sun" symptom, unstable horizontal nystagmus, and descending strabismus are noted. Muscular dystonia is detected, more in the distal parts of the extremities in the form of a symptom of “seal feet” and “heel piles”. In most children, especially in the first days of life, these phenomena are combined with paroxysms of startling, spontaneous Moro reflex, sleep disturbance, Harlequin's symptom, general and local cyanosis. The development of hypertensive-hydrocephalic syndrome on the 3rd - 5th day of life may be a sign of periventricular hemorrhage. Hypertensive-hydrocephalic syndrome can be isolated, but is more often combined with depression syndrome or coma syndrome. The depression syndrome
is manifested by lethargy, physical inactivity, decreased spontaneous activity, general muscle hypotonia, hyporeflexia, suppressed reflexes of newborns, decreased sucking and swallowing reflexes.
Local symptoms are observed in the form of divergent and convergent strabismus, nystagmus, asymmetry and sagging of the lower jaw, asymmetry of the facial muscles, bulbar and pseudobulbar symptoms. The syndrome characterizes the course of the acute period of hypoxic encephalopathy and usually disappears at the end of the first month of life. In the acute period, depression syndrome can be a harbinger of cerebral edema and the development of coma. Comatose syndrome
is a manifestation of the serious condition of the newborn; the Apgar scale is estimated at 1 - 4 points.
The clinical picture reveals severe lethargy, adynamia, muscle hypotonia to atony, congenital reflexes are not detected, the pupils are constricted, the reaction to light is insignificant or absent. There is no reaction to painful stimuli, “floating” movements of the eyeballs, horizontal and vertical nystagmus, tendon reflexes are depressed. Breathing is arrhythmic, with frequent apnea, bradycardia, muffled heart sounds, arrhythmic pulse, low blood pressure. There may be attacks of convulsions with a predominance of the tonic component. The severe condition persists for 10 - 15 days, there are no sucking and swallowing reflexes. The appearance of hydrocephalus in the acute period with bulging and tension of the large fontanel, divergence of cranial sutures, protrusion of the eyeballs, and rapid growth of the head indicates intracranial hemorrhage. Convulsive syndrome
in the acute period is usually combined with depression or coma syndrome.
Occurs as a result of hypoxic cerebral edema, hypoglycemia, hypomagnesemia, or intracranial hemorrhage. Manifests itself in the first days of life with tonic-clonic or tonic convulsions. Along with this, local clonic convulsions or hemiconvulsions are observed. Convulsive seizures in newborns are characterized by short duration, sudden onset, lack of pattern of repetition and dependence on the state of sleep or wakefulness, feeding regimen and other factors. Convulsions are observed in the form of small-amplitude tremor, short-term cessation of breathing, tonic spasm of the eyeballs such as upward gaze paresis, imitation of the “setting sun” symptom, nystagmus, automatic chewing movements, paroxysms of foot clonus, vasomotor reactions. These convulsions are sometimes similar in nature to the spontaneous movements of a child, which makes diagnosis difficult. The recovery period of hypoxic encephaloratia includes the following syndromes: increased neuro-reflex excitability, hypertensive-hydrocephalic, vegetative-visceral dysfunction, motor disorders, delayed psychomotor development, epileptic. The syndrome of increased neuro-reflex excitability in the recovery period has two course options. With a favorable course, there is a disappearance or decrease in the severity of symptoms of increased neuro-reflex excitability within a period of 4-6 months to 1 year. In unfavorable cases, especially in premature infants, epileptic syndrome may develop. Hypertensive-hydrocephalic syndrome has two variants of the course: 1) hypertensive-hydrocephalic syndrome with a favorable course, in which there is a disappearance of hypertensive symptoms with a delay in hydrocephalic ones; 2) an unfavorable variant of hypertensive-hydrocephalic syndrome, which is part of the symptom complex of organic cerebral syndrome. Outcomes of hypertensive-hydrocephalic syndrome: 1. Normalization of head circumference growth by 6 months. 2. Compensated hydrocephalic syndrome at 8 - 12 months. 3. Development of hydrocephalus. The syndrome of vegetative-visceral dysfunctions
begins to manifest itself after 1 - 1.5 months of life against the background of increased neuro-reflex excitability and hypertensive-hydrocephalic syndrome.
The clinical picture includes persistent regurgitation, persistent malnutrition, respiratory rhythm disturbances and apnea, changes in skin coloration, acrocyanosis, paroxysms of tachy- and bradypnea, thermoregulation disorders, gastrointestinal dysfunction, and temporal baldness. Epileptic syndrome
can manifest itself at any age (as a continuation of seizures after birth or against the background of a somatic infection).
In newborns and infants, it has the so-called age face, i.e. convulsive paroxysms imitate the motor capabilities that the child possesses at the time of their appearance. In newborns and infants (especially premature infants), convulsive syndrome is characterized by a variety of clinical forms of seizures. Generalized convulsive seizures (tonic-clonic, clonic, tonic), abortive, focal, hemiconvulsive, polymorphic seizures, simple and complex absence seizures are observed. Polymorphic forms of seizures predominate in frequency. In premature infants with perinatal encephalopathy, propulsive and impulsive paroxysms do not occur in isolated form, but are observed only as part of polymorphic seizures. The greatest difficulty in diagnosis is abortive and non-convulsive forms of paroxysms. An imitation of unconditioned motor reflexes is observed in the form of paroxysmal manifestations of a cervical-tonic symmetrical reflex with a tilt of the head and tonic tension of the arms and legs; cervical-tonic asymmetric reflex with turning the head to the side and extension of the same arm and leg; the first phase of the Moro reflex with the opening of the arms. There are paroxysms in the form of gaze spasms and nystagmus, an imitation of the “setting sun symptom.” Attacks of redness and blanching of the skin with increased sweating and sometimes regurgitation are often observed. After 3-4 months of life, as the ability to hold the head appears, “nods” and “nods” appear, and from 6-7 months - “bows” (bending the body back and forth). Such features of the convulsive syndrome in premature infants, such as instability of clinical manifestations with a predominance of polymorphic seizures, the presence of abortive forms of seizures, as well as complex absences with imitation of unconditioned reflexes of newborns (the first phase of the Moro reflex, asymmetric cervical-tonic reflex) are probably a consequence of the immaturity of the structures brain. However, an increase in the frequency of seizures, an increase in the polymorphism of their manifestations, and resistance to anticonvulsant therapy should be alarming regarding the formation of gross organic forms of brain damage. Polymorphism of attacks and their resistance to therapy is an unfavorable prognostic sign. The syndrome of movement disorders
is detected from the first weeks of life and can occur with muscle hypotension or hypertension. When a syndrome of motor disorders with muscle hypotonia appears, there is a decrease in spontaneous motor activity, inhibition of tendon reflexes and congenital unconditioned reflexes of newborns. The syndrome of motor disorders with muscle hypotension occurs in isolation, as well as in combination with hypertensive-hydrocephalic syndrome, a syndrome of increased neuro-reflex excitability with a decrease in the threshold of convulsive readiness. The combination of movement disorder syndrome and convulsive syndrome is unfavorable. The increase in muscle hypertension in full-term infants and the appearance of side-by-side focal symptoms should raise alarm bells regarding the development of cerebral palsy. The syndrome of delayed psychomotor development begins to manifest itself from 1 to 2 months. In the structure of the syndrome, there is a violation of the reduction of unconditioned congenital reflexes. The cervical-tonic symmetrical and asymmetrical reflexes, the delay in the formation of rectifying labyrinthine chain tonic reflexes, acquire a greater diagnostic role. If the structure of this syndrome includes mental retardation, children by the age of one month experience insufficiently stable gaze fixation, short-term tracking with rapid exhaustion. There is no reaction to the mother's voice, auditory concentration. By 2 - 3 months of age, there is insufficient animation during communication, the cry is inexpressive, there is no humming, children look for the source of sound with their eyes without turning their heads, and a rare, difficult to evoke smile appears. By six months - they are not actively interested in toys and surrounding objects, they do not react sufficiently to the presence of the mother, the humming is inactive and short-lived, manipulations with objects are delayed, there is no active attention. If the developmental delay is “tempo”, it begins to disappear with proper nursing. At the age of 4-5 months, this group of children becomes more active as if in a “leap”, and mental development is ahead of motor development. An emotional reaction to others and an interest in toys appear. Age-related motor functions begin to be actively compensated after 6–7 months and, as a rule, are restored by 1–1.5 years. Long-term mental retardation is unfavorable prognostically.
Diagnostics
Examination of the fundus in the acute period of mild hypoxic encephalopathy does not reveal any abnormalities; moderate congestion of the veins is observed less frequently. In moderate cases, varicose veins, swelling, and isolated hemorrhages are noted. In severe cases of damage, against the background of pronounced edema of vasodilation, blurring of the boundaries of the optic nerve head and hemorrhage are noted. In the future, such children may develop atrophy of the optic nerves. Changes in the cerebrospinal fluid are detected in the presence of intracranial hemorrhage. In these cases, the cerebrospinal fluid contains fresh and leached red blood cells. After the 7th - 10th day of life, confirmation of hemorrhage is the presence of macrophages in the cerebrospinal fluid. Neurosonography
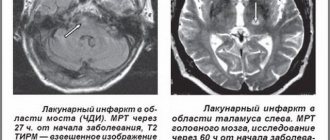
- two-dimensional ultrasound examination of the anatomical structures of the brain through the large fontanel - allows you to establish periventricular hemorrhage, foci of leukomalacia, expansion of the ventricular system - ventriculomegaly [5, 10].
Neurosonography allows for differential diagnosis with brain defects. Signs of brain hypoplasia: enlargement of subarachnoid spaces, widening of the interhemispheric fissure, ventriculomegaly, increased density in the area of the brain parenchyma without clear differentiation of convolutions, porencephaly. Haloprosencephaly is an enlargement of one ventricle, shadows, increased echo density from brain stem structures, decreased density from brain parenchyma [7]. Clinical neurosonographic comparisons reveal a correlation between the number of leukomalacia and neurological outcomes. Multiple leukomalacia in both hemispheres, detected in patients according to neurosonography in the acute period of the disease, is combined with a syndrome of motor disorders and severe retardation of psychomotor development in the recovery period. Computed tomography of the brain helps to objectify hypoxic changes in brain tissue in the structures of the cerebellum and brain stem, which are not clearly identified by neurosonography. An electroencephalographic
(EEG) study reveals foci of slow-wave activity, foci of reduction of cortical rhythm, and foci of epileptic activity. An EEG study is of great importance for identifying a risk group for convulsive syndrome and diagnosing clinically “silent” seizures. An indirect confirmation of convulsive syndrome, as well as a sign of a lowered threshold of convulsive readiness in patients, is the presence of paroxysmal EEG changes. Repeated seizures can lead to increased severity of paroxysmal activity on the EEG. From biochemical studies in the acute period, an indicator of the depth of hypoxic disorders is the assessment of acidosis from mixed to severe decompensated metabolic. With severe hypoxia, the osmotic pressure of the blood plasma increases and the level of lactate dehydrogenase increases. Lactate dehydrogenase and other glycolytic enzymes correlate with the severity of the condition of children and reflect the severity of hypoxia in the acute period of perinatal encephalopathy. X-ray of the lungs is used to diagnose congenital atelectasis, pneumopathy and inflammatory changes in the lungs.
Treatment of the acute period
In the acute period, timely correction of respiratory distress syndrome and adequate ventilation
. In premature infants, colfosceryl palmitate 5 ml/kg is administered endotracheally. The use of surfactant analogs leads to a significant regression of neurological syndromes of hypoxic encephalopathy. 1. Correction of homeostasis and hypovolemia: fresh frozen plasma 5 - 10 ml/kg, 10% albumin 5 - 10 ml/kg, rheopolyglucin 7 - 10 ml/kg, hemodez 10 ml/kg. 2. Reducing vascular permeability: 12.5% etamsylate solution intramuscularly or intravenously, 1% Vicasol 0.1 ml/kg. 3. Metabolic and antioxidant therapy: piracetam 50 mg/kg, 10% glucose 10 ml/kg, actovegin intravenously, 5% vitamin E 0.1 ml per day. Soybean oil is used as an antioxidant: 2 - 3 ml for 4 - 6 days on the skin of the abdomen [11]. 4. Vascular therapy: vinpocetine 1 mg/kg intravenously. 5. Dehydration therapy: hydrocortisone 3 - 10 mg/kg, prednisolone 1 - 2 mg/kg, 25% magnesium sulfate 0.2 ml/kg. 6. Improvement of tissue metabolism of the heart muscle: cocarboxylase 8 mg/kg, ATP 10 mg/kg. 7. Anticonvulsant therapy: diazepam 1 mg/kg intramuscularly or intravenously, GHB 50 mg/kg, barbiturates, when benzodiazepines are not effective, 5 mg/kg [14].
Treatment of the recovery period
Treatment during the recovery period is carried out according to the syndromic principle. 1. For the syndrome of increased neuro-reflex excitability with manifestations of vegetovisceral dysfunction, sedatives are indicated: diazepam 0.001 g 2 times a day, tazepam 0.001 g 2 times a day, medicine with citral - citral solution 2.0, magnesium sulfate 3.0, 10 % glucose solution 200.0 - a teaspoon 3 times a day; from 2 months of age, a soothing cocktail of herbs (valerian root, motherwort, sage) is prescribed, 1 teaspoon 3 times a day. 2. For hypertensive-hydrocephalic syndrome, it is advisable to prescribe furosemide 0.002 g/kg per day with panangin, glycerol 1 teaspoon 3 times a day. For severe manifestations of hypertensive-hydrocephalic syndrome, use acetazolamide 0.02 g/kg per day once according to the following scheme: 3 days on, 1 day off, course from 3 weeks to 1 - 1.5 months with panangin. 3. For movement disorder syndrome: vitamin B6 5 mg, vitamin B1 2 mg, ATP 0.5 ml 10 - 12 injections, pyritinol 10 - 20 drops per 1 kg of body weight 2 times a day in the morning for 1 - 3 months. Massage. Therapeutic gymnastics, training of the mother in rehabilitation skills. 4. For psychomotor development delay syndrome: piracetam 30 - 50 mg/kg in 3 doses, pyritinol. From 6 months Cerebrolysin 0.5 ml No. 20 (contraindicated in convulsive syndrome), vitamins B6, B1. Gamma-aminobutyric acid 0.06 g 2 - 3 times a day. 5. For convulsive syndrome: sodium valproate 20 - 50 mg/kg, clonazepam up to 1 - 2 mg per day, phenobarbital 1 - 2 mg/kg. For resistant seizures, lamotrigine 1 - 2 mg/kg. 6. Surgical treatment is used for combined periventricular hemorrhages and the development of posthemorrhagic hydrocephalus; ventricular shunting is performed. Therapeutic measures are further individualized depending on the outcome of the process. By the age of one, in most mature children, with the appearance of verticalization and speech functions, the outcomes of perinatal hypoxic encephalopathy can be identified. Recovery occurs in 15 - 20% of children. Frequent consequences of encephalopathy are minimal brain dysfunction and hydrocephalic syndrome. The most severe outcomes are cerebral palsy and epilepsy. Of course, advances in perinatal medicine, adequate management of childbirth and the acute period of hypoxic encephalopathy will reduce the neuropsychiatric outcomes of the disease.
Literature:
1. Balan P.V., Maklakova A.S., Krushinskaya Ya.V., Sokolova N.L., Kudakov N.I. Comparative analysis of resistance to acute hypobaric hypoxia in newborn and adult experimental animals. Obstetrics and gin. 1998;3:20-3. 2. Gromyko Yu.L. Evaluation of the effectiveness of a new antioxidant drug, Actovegin, for the treatment of placental insufficiency and retardation in fetal size. Materials of the 1st Congress of the Russian Association of Perinatal Medicine Specialists, 32. 3. Ivanovskaya T.E., Pokrovskaya L.Ya. The main pathology of the perinatal period according to modern pathological data. Pediatrics 1987;4:11-7. 4. Kulakov V.I. Prenatal medicine and women's reproductive health. Obstetrics and gin. 1997;5:19-22. 5. Kuznetsova L.M., Dvoryakovsky I.V., Mordova N.A. Correlation of clinical and ultrasound signs in liquorodynamic disorders in young children. Materials of the scientific and practical conference. Kaluga, 1980;34. 6. Milenin O.B., Efimov M.S. The use of synthetic surfactant exosorf in the treatment and prevention of respiratory distress syndrome in newborns. Obstetrics and gin. 1998;3:5-9. 7. Orlova N.S., Machinskaya E.A., Fishkina E.V. Neurosonography in the diagnosis of certain malformations of the brain. Materials of the scientific and practical conference. Kaluga. 1982;35. 8. Pediatrics (English translation) / Ed. N.N. Volodina. M., 1996;125-70. 9. Fedorova M.V. Placental insufficiency. Obstetrics and gin. 1997;5:40-3. 10. Fishkina E.V., Simushin G.P., Rubtsova I.I. and others. Possibilities of neurosonography in the diagnosis of lesions of the central nervous system in newborns. Materials of the scientific and practical conference. Kaluga. 1980;43. 11. Sharipov R.Kh. The use of membranotropic drugs in the complex treatment of premature infants with perinatal encephalopathy. Abstracts of reports of the scientific-practical conference. Samara. 1993;1:63. 12. Edelshtein E.A., Bondarenko E.S., Bykova L.I. Perinatal hypoxic syndromes. Tutorial. M., 1988;38. 13. De Volder AG, Joffinet AM, Bol A, et al. Brain glucose metabolism in postanoxic syndrome. Arch Neurol 1990;47(2):197-204. 14. Carlier G, Guidi O, Dubru JM. Le traitement des convulsions d'enfant. Rev/med/ Liege. 1989;257-62. 15. J. VoIpe Neurology of the Newborn, coundres company. 1987;715.
Diagnostic principles
The diagnosis is based on objective symptoms, results of laboratory and instrumental examinations and anamnesis. In addition to the mandatory examination by a therapist and neurologist, the following are indicated:
- general clinical analysis of blood and urine;
- biochemical blood test with determination of glucose, ALT, AST, bilirubin, urea, creatinines and electrolytes;
- according to indications: analysis to determine immunospecific status, neurospecific factors;
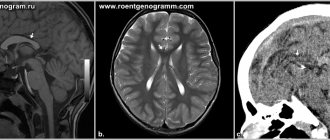
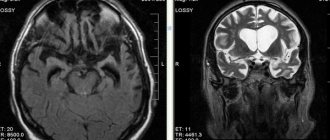
- CT, MRI, angiography;
- specific tests to detect heavy metals and narcotic substances in the body.
If symptoms indicate damage to internal organs, additional research or consultation with specialized specialists may be required. Differential diagnosis is carried out with other forms of encephalopathies and neurodegenerative diseases.
Cost of services
| CONSULTATIONS OF SPECIALISTS | |
| Initial consultation with a psychiatrist (60 min.) | 6,000 rub. |
| Repeated consultation | 5,000 rub. |
| Consultation with a psychiatrist-narcologist (60 min.) | 5,000 rub. |
| Consultation with a psychologist | 3,500 rub. |
| Consultation with Gromova E.V. (50 minutes) | 12,000 rub. |
| PSYCHOTHERAPY | |
| Psychotherapy (session) | 7,000 rub. |
| Psychotherapy (5 sessions) | 30,000 rub. |
| Psychotherapy (10 sessions) | 60,000 rub. |
| Group psychotherapy (3-7 people) | 3,500 rub. |
| Psychotherapy session with E.V. Gromova (50 minutes) | 12,000 rub. |
| TREATMENT IN A HOSPITAL | |
| Ward for 4 persons | 10,000 rub./day |
| Ward for 3 persons | 13,000 rub./day |
| Ward 1 bed VIP | 23,000 rub./day |
| Individual post | 5,000 rub. |
| PETE | 15,000 rub./day |
This list does not contain all prices for services provided by our clinic. The full price list can be found on the “Prices” , or by calling: 8(969)060-93-93. Initial consultation is FREE!
Clinical guidelines for the treatment of toxic encephalopathy
The basic principles of disease treatment are as follows:
- restoration and maintenance of the full functioning of internal organs and systems;
- stopping further entry of the neurotoxin into the body (limiting work activity, giving up alcohol and/or drugs, etc.);
- speeding up metabolism to eliminate poison as quickly as possible, using specific antidotes;
- symptomatic treatment.
In the acute period, therapy is carried out strictly in a hospital setting under round-the-clock medical supervision. As the patient’s well-being improves, the patient is transferred to an outpatient regimen. For a long course the following is prescribed:
- nootropic drugs;
- drugs that improve metabolic processes in the brain;
- neuropeptides;
- antioxidants;
- means for normalizing the tone of cerebral vessels;
- vitamins and dietary supplements (group B, nicotinamide, riboxin, succinic, ascorbic acid, glycine, etc.);
- if necessary, tranquilizers from the group of benzodiazepines, antidepressants, sleeping pills.
Symptoms of toxic encephalopathy are a mandatory indication for contacting a neurologist. No treatment with folk remedies - only comprehensive, individually selected therapy in combination with physical procedures, dietary correction, and lifestyle changes. Don't hesitate to start therapy! To make an appointment or call a specialized specialist at home, call us 24/7 at 8(969)060-93-93.
Chronic alcoholic encephalopathies
Korsakov's psychosis is more often observed in women and is manifested by memory impairment, false memories and disorientation. Patients remember new information poorly and have difficulty remembering what happened to them before the onset of the disease. In conversations with patients, it turns out that they often “remember” events that did not actually happen. Orientation in space, place and time is difficult. There is a lack of speech and motor reactions. Neurological disorders in the form of neuritis are detected. With the abolition of alcohol, the symptoms of the disease may be reduced.
Alcoholic pseudoparalysis usually occurs in men. Both gradual development and rapid progression of symptoms after acute alcoholic psychosis are possible. Dementia is characterized by loss of previous knowledge and skills, memory disorders and decreased criticism of one’s condition. There is coarsening (rudeness, cynicism) combined with sudden mood swings. Neurological symptoms are represented by polyneuritis, speech disorders, trembling of the muscles of the hands and face.