A.Yu. Lubnin, A.A. Potapov, I.V. Nikitenkova, I.A. Savin, K.A. Popugaev, A.V. Oshorov
FGAU "National Medical Research Center of Neurosurgery named after. acad. N.N. Burdenko" of the Ministry of Health of Russia, Moscow, Russia
For correspondence: Lubnin Andrey Yurievich - Dr. med. Sciences, Professor, Head of the Department of Anesthesiology, Resuscitation and Intensive Care of the Federal State Institution "National Medical Research Center of Neurosurgery named after. acad. N.N. Burdenko" of the Ministry of Health of Russia, Moscow; e-mail
For citation: Lubnin A.Yu., Potapov A.A., Nikitenkova I.V., Savin I.A., Popugaev K.A., Oshorov A.V. Sudden cerebral edema after uncomplicated bilateral cranioplasty. Clinical observation and literature review. Bulletin of Intensive Care named after. A.I. Saltanova. 2020;2:137–145. DOI: 10.21320/1818-474X-2020-2-137-145
Essay
The paper describes a clinical observation of the acute development of cerebral edema after uncomplicated bilateral cranioplasty. The discussion and review of the literature examines the mechanisms of development of cerebral edema that occurs after the closure of extensive bone defects of the skull, especially large and bilateral ones, as well as the possible role of subgaleal drainages.
Key words: cranioplasty, complications, acute cerebral edema
Received: 03.03.2020
Accepted for publication: 02.06.2020
Read article in PDF
Statistics Plumx Russian
Introduction
Decompressive craniectomy (DC) is a fairly frequently performed neurosurgical operation aimed mainly at eliminating severe intracranial hypertension resistant to therapeutic measures in the acute period in patients with severe traumatic brain injury and cerebral vascular accidents (malignant ischemic stroke, severe aneurysmal subarachnoid hemorrhage, sinus thrombosis) [1–9]. The consequence of DC, if the patient is experiencing an acute period of catastrophe, is the presence of extensive bone defects of the cranial vault, sometimes bilateral, which poses a certain danger for these patients and creates cosmetic problems. To close bone defects after DC, plastic surgery is usually performed to close these defects using autologous bone (if it can be preserved), various synthetic plastic materials or metal plates [10–12]. This operation is usually performed delayed, when the patient's condition is already stable; as a rule, it does not present any technical difficulties and is not accompanied by any complications. However, in our practice, we were faced with a rare situation - the sudden development of massive cerebral edema in the immediate postoperative period after uncomplicated bilateral cranioplasty. Below is a description of this observation.
Symptoms for various degrees of TBI severity
The severity of TBI is determined by the Glasgow Coma Scale (assessing the patient’s eye opening, movement and speech): mild – 13–15 points, moderate – 9–12 points, severe – 3–9 points. In other words, three factors are taken into account: state of consciousness, vital functions, neurological symptoms. In this case, the patient’s condition is assessed as satisfactory, moderate, severe, extremely severe, terminal (between life and death). The more serious the injury and the deeper its penetration, the more difficult the patient’s recovery.
Symptoms of TBI with:
- Mild severity: headache (cephalalgia), dizziness, nausea and vomiting, sleep disturbance, irritability, fatigue.
- Moderate: speech impairment, partial loss of vision, paroxysms of the limbs, mental disorders, memory loss, heart rhythm disturbances, high-intensity cephalgia, increased frequency of respiratory movements while maintaining rhythm, loss of consciousness for up to several hours.
- Severe: convulsions, paralysis, hypo- or hypertonicity of muscles, lack of consciousness for up to several weeks, coma, critical impairment of vital functions, deep brainstem and cerebral disorders.
Clinical observation
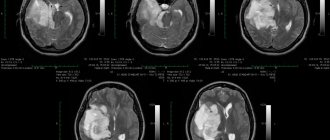
Patient T., 18 years old, was admitted to our clinic with a diagnosis of the consequences of a severe combined traumatic brain injury, an emerging vegetative state, and bilateral bone defects of the cranial vault. From the anamnesis it is known that for 8 months. Before this hospitalization, as a result of a traffic accident (car accident), the patient received a severe traumatic brain injury: severe brain contusion with an open fracture of the facial bones, rotational dislocation in the right intervertebral joint C1–C2 and subluxation of the atlantodentate joint. She was taken in extremely serious condition to the nearest hospital, where she underwent bilateral DC on the same day in the frontotemporo-parietal regions. Subsequently, the patient received the full range of intensive care measures used in victims with severe traumatic brain injury, including tracheo- and gastrostomy, installation of a ventriculoperitoneal shunt due to the development of post-traumatic hydrocephalus. The result of the treatment was the formation of a vegetative state. After 3 months from the moment of injury, against the background of the patient’s stable condition, she was transferred at the insistence of relatives to a rehabilitation clinic in Israel. The treatment carried out there did not produce any special results, but, according to relatives, at the beginning of treatment, for some unknown reason, the patient developed a short-term episode of asystole, which was relieved by resuscitation measures. At the time of admission to our clinic, the patient’s condition was serious, in a vegetative state, there was no contact with her, she lay with her eyes periodically open. Massive bilateral bone defects as a result of previously performed DC (Fig. 1). Breathing independently, through a tracheostomy. Eating through a gastrostomy tube, urinating through a permanent urinary catheter. The purpose of hospitalization in our center is to close bone defects of the skull.
Rice. 1. Preoperative CT scans of patient T.
Large bilateral calvarial defects and a shunt are visible.
Fig. 1. Preoperative CT scan of patient T.
After standard preoperative preparation, the patient was taken to the operating room for cranioplasty. Anesthetic management: IV propofol, as a continuous infusion + small doses of midazolam at the beginning of the operation + fentanyl bolus during the operation. Muscle relaxation - rocuronium bromide. Artificial pulmonary ventilation (ALV) through a tracheostomy with an oxygen-air mixture (FiO2 = 0.3) in normal ventilation mode. The course of anesthesia is smooth, blood loss during the entire operation is no more than 100 ml, infusion therapy is crystalloids.
The scope of the surgical intervention, as planned, was reduced to plastic closure of extensive bilateral bone defects of the cranial vault using a stereolithographic model of the skull and implant molds. The operation was completed by suturing the soft tissue in the area of the surgical wounds with the installation of subgaleal active drainages under the skin on both sides. At the end of the operation, the patient on mechanical ventilation with an Ambu bag was transferred to the recovery room, where mechanical ventilation in the SIMV mode and monitoring of basic physiological parameters were continued.
Everything was calm, but an hour and a half after the end of the operation, the patient showed a tendency towards arterial hypotension and tachycardia, but the most unpleasant thing was that at the same moment the appearance of bilateral mydriasis was noted, indicating some kind of acutely developed intracranial catastrophe. The decrease in blood pressure was corrected by IV infusion of vasopressors, and the patient was transferred to the intensive care unit. There she immediately underwent a control CT scan of the brain, which showed severe diffuse edema of all brain structures, including the posterior cranial fossa. The reason for such acutely developed massive cerebral edema after uncomplicated cranioplasty remained not entirely clear, but given the high probability of severe intracranial hypertension against the background of such cerebral edema, it was decided to immediately remove the installed bone implants and subgaleal drainages, which was done in the operating room.
The patient then underwent intensive therapy in the intensive care unit. On the second day after surgery, the patient underwent magnetic resonance imaging of the brain, which confirmed the presence of severe diffuse edema of the medulla in all parts of the brain (Fig. 2). In addition, multiple small hemorrhages were found in almost all parts of the brain. There were no signs of thrombosis of the main arterial and venous vessels of the brain.
Rice. 2. Magnetic resonance imaging of patient T., performed on the 2nd day after surgery
Pronounced diffuse edema of the medulla in all parts of the brain and small pinpoint hemorrhages are visible.
Fig. 2. MRI patient T., performed on the 2nd day after surgery
During intensive therapy, the patient's condition gradually stabilized. Systemic hemodynamics are stable, and there is no need for vasopressor support. Ventilation through a tracheostomy in auxiliary mode. Infusion therapy through a central venous catheter, nutrition through a gastrostomy tube, diuresis through a permanent urinary catheter. Electroencephalography recorded on the 4th day after surgery revealed a low level of bioelectrical activity: the alpha rhythm is not recorded, frequent fluctuations in the beta range are recorded in the central frontal areas, more on the right. Slow waves of the delta range of low amplitude, polymorphic in nature, are noted in the right hemisphere, more in the central frontal anterior temporal region. No reaction to afferent stimulation (rhythmic light, sound, pain stimulation) was obtained. Neurologically - a vegetative state, without any dynamics. This condition of the patient persisted for four and a half years: she remained a patient in the intensive care unit in our clinic, mainly at the insistence of relatives. After this period of time, the patient died from severe respiratory problems.
Preparation for craniotomy
If there is enough time for preparation, the patient undergoes a comprehensive examination.
However, in the case of emergency trepanation, a minimum set of tests is performed and internal diseases are ignored in favor of saving life.
A week before surgery, the patient stops taking anticoagulants, and the day before, he stops eating and drinking.
During the procedure, the patient is placed on the operating table, the head is fixed, and anesthesia is administered. The hair in the desired area is shaved, the skin is cut and separated from the skull.
Holes are drilled in the skull, their contours are rounded and the cut out part is removed. Then the dura mater is removed to the side. Further tactics depend on the purpose of the surgical intervention.
Upon completion of the manipulations on the brain body, which sometimes take many hours, the meninges and the cut out piece of bone are returned to their place, which is secured with titanium plates. The skin is sutured on top.
Discussion
Cranioplasty is a fairly frequently performed neurosurgical intervention. Most often, it is performed to eliminate the consequences of DC in victims with severe traumatic brain injury or patients with cerebral vascular accidents [1–9]. Cranioplasty does not involve any impact on the brain matter, and even the dura mater is not opened during this operation. The condition of patients undergoing this operation is usually stable, since it is performed in the delayed period of injury or vascular accident. Therefore, cranioplasty is rarely complicated by any serious problems. All the more incredible is the situation we encountered in our clinical practice. What happened to our patient an hour and a half after the end of the uncomplicated and, in fact, extra-cerebral operation? We have repeatedly discussed this clinical situation, and a variety of assumptions have been made.
- Compression of the trunk due to rotational dislocation in the right intervertebral joint at the level of C1–C2 and subluxation of the atlantodentate joint, presumably occurring during the injury? But this is an old process, correlated in time with the moment of injury. The patient did not undergo tracheal intubation in the operating room, which means there was no moment of extension of the cervical spine, which could be considered as a provoking factor for acute compression of the cervical spinal cord (extension against the background of muscle relaxation - elimination of the “muscular frame”) [13, 14]. Further, our patient’s cerebral catastrophe did not develop at the time of transfer, which could also provoke compression. And finally, even if we assume a situation of compression of the cervical spinal cord, this in no way explains the rapid development of total (hemispheric + structure of the posterior cranial fossa) cerebral edema.
- Thrombosis of the main arterial and venous vessels of the brain (primarily venous sinuses). In principle this is, of course, possible. But the reason for this complication in our patient is not obvious. The most common cause of sinus thrombosis is thrombophilia, especially in combination with the prescription of hormonal contraceptives, and a local inflammatory process [15–20]. These situations were absent in our patient. Thrombosis of the cerebral sinus, especially large and unpaired (sagittal type), manifests itself with the formation of a hematoma or two symmetrical ones in the parasagittal region [16, 17], which also did not occur in our patient. But the main thing is that the diagnosis of sinus thrombosis was not confirmed by neuroimaging.
- Acute cerebrovascular accident in the form of cerebral hyperperfusion after cranioplasty, especially bilateral, in combination with the installation of two active subgaleal drainages. At first glance, this explanation seems complex and obscure, but let’s try to understand it.
The first factor is cranioplasty. Despite its apparent simplicity and relative non-invasiveness, cranioplasty turns out to have a pronounced effect on cerebral hemodynamics, even unilaterally. As has been shown in a series of numerous clinical studies, sealing the cranial cavity causes a significant increase in linear and volumetric cerebral blood flow values (the latter effect may be the result of eliminating the effect of atmospheric pressure) [21–28]. The mechanism of this effect remains unclear, but perhaps it is the reason for the progressive clinical improvement in the neurological status of these patients after sealing the cranial cavity and eliminating the effect of atmospheric pressure [21–23, 29–33].
The second factor is active subgaleal drainages. The accumulation of blood in the area of the surgical wound under the skin flap is a minor, but unpleasant complication of any neurosurgical operation. These simple, cheap and effective devices were developed to prevent such accumulation. However, an analysis of the literature data provided quite alarming information: it turns out that in some observations, the use of active subgaleal drainage led to such serious complications as the formation of an intracranial hematoma, pseudohypoxic cerebral edema, rupture of an incompletely clipped aneurysm and axial dislocation in the cranial direction, accompanied by reflex asystole [ 34–41]. It is believed that the negative pressure created by active subgaleal drainage can be transmitted in a certain way into the cranial cavity, creating conditions there for intracranial hypotension with all the ensuing consequences [34, 37–39]. In our observation, two such drains were used, and by the time mydriasis was detected, both were almost full, and the capacity of each “pear” was at least 100–150 ml.
It can be assumed that the effects of bilateral cranioplasty and active subgaleal drainage could be summed up and create real conditions for an acute increase in volumetric cerebral blood flow in our patient, and the further development of total cerebral edema and multiple hemorrhages was a consequence of this cerebral hyperemia.
Analysis of literature data showed that our observation is not unique and a number of similar clinical situations have been described in the world [40, 42–46]. They describe isolated clinical observations of the development of severe cerebral edema after uncomplicated cranioplasty. The authors of these works, like us, do not have an absolute evidence base explaining the development of this complication, but the course of their reasoning in an attempt to find an explanation for this phenomenon is close to ours.
The situation with our clinical observation and other similar ones was significantly changed and supplemented by a meta-analysis conducted by Mexican authors and published in World Neurosurgery in March 2021 [47]. This relatively recent publication explains a lot, although not everything, and therefore, in our opinion, it is worthy of more detailed consideration.
The authors of the analysis focused their attention on the problem of MBSC (Massive Brain Swelling after Cranioplasty). Unfortunately, even terminologically, not everything is so simple here, and the authors rightly point out that other definitions exist and are used for this condition in the literature: “death after cranioplasty”; “cerebral edema after cranioplasty”, etc. Nevertheless, the authors did a great job. Using several medical databases from 1960 to 2017, they selected relevant publications (though only in English), in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. At first, the selected works were 534 (mainly descriptions of single clinical observations or small series), then their number was significantly reduced (to 19), and this made it possible to summarize the already fairly representative material from 26 patients.
The age of the patients ranged from 14 to 77 years. The reasons for decompressive trepanation were traumatic brain injury (52%), acute cerebrovascular accident (44%), but in older patients acute cerebrovascular accident was still the leading cause. The type of craniotomy was as follows: in 21 patients (81%) it was hemicraniectomy, in the rest it was bilateral craniectomy. The shunt was implanted in 11 patients (44%) at the time of cranioplasty. According to the type of cranioplastic material - in 50% of cases it was autologous bone, in the rest - various plastic materials.
The time indicators are of interest. The time of cranioplasty from the moment of development of cerebral catastrophe varied from 1 to 17 months. (on average 3 months). The time from the end of the cranioplasty operation to the development of cerebral edema varied from 15 minutes to 16 hours (on average 3.3 hours).
Clinical manifestations . The most common were mydriasis and coma. Seizures developed in 30% of patients. The radiological picture was the same in all patients: diffuse cerebral edema with compression of the reserve liquor spaces. In some observations, signs of intraparenchymal hemorrhage were noted.
And finally, treatment and outcomes. The most common surgical option (65% of patients) was immediate removal of bone implants. However, some patients received decongestant therapy, while others (n = 4) did not receive any treatment. At the same time, the mortality rate was 88%, and the three surviving patients remained deeply disabled. These are the data from the analysis of this series of observations of 26 patients. What does this information suggest?
- Despite all its relative technical simplicity and minimally invasiveness (after all, during cranioplasty even the dura mater is usually not opened), cranioplasty after decompressive craniectomy is pathophysiologically a serious intervention, leading to a sharp increase in volumetric cerebral blood flow and cerebral metabolism. The reasons for this increase in cerebral blood flow are most likely related to the sealing of the cranial cavity, which leads to the elimination of the effect of atmospheric pressure on cerebral blood flow. This abrupt increase in cerebral blood flow, especially against the background of impaired mechanisms of its autoregulation, essentially leads to the development of cerebral hyperperfusion syndrome, which is well known and has been repeatedly described in other clinical conditions - carotid endarterectomy, endovascular revascularization interventions, resection of large hemispheric arteriovenous malformations with large discharge blood [48–55]. It is interesting that the clinical manifestations of cerebral hyperperfusion syndrome in all these conditions are quite the same: psychomotor agitation or delirium, convulsions, cerebral edema and hemorrhages in its substance.
- The presence of a functioning shunt and external drainages. These factors are clearly considered by most authors to be high risk on the basis that, although in different ways, they all lead to a decrease in intracranial pressure and thereby contribute to an increase in volumetric cerebral blood flow.
- Considering the preoperative presence of severe neurological symptoms and the rapid rate of development of cerebral edema during cranioplasty operations after decompressive craniectomy, the anesthesiologist should be extremely attentive to all systemic changes in hemodynamics (the development of tachy- or bradycardia, arterial hyper- or hypotension), both during the operation and in the immediate postoperative period. And of course, it is necessary to evaluate the dynamics of neurological symptoms and especially the appearance of mydriasis.
- Immediate removal of established bones or bone substitutes and decongestant therapy appear to be reasonable treatment options in this situation. However, treatment outcomes most often remain poor. Preventative measures are not yet fully understood. Perhaps this is a refusal to use active drainages; temporary or permanent blockade of the liquor shunt; staged rather than one-stage closure of large and bilateral defects of the skull bones.
NSICU.RU neurosurgical intensive care unit website of the intensive care unit of the N.N. Research Institute Burdenko
After removal of brain tumors, brain edema may develop and intracranial pressure (ICP) may increase. One of the probable and least studied reasons for this is a violation of venous outflow. The severity of clinical manifestations and outcomes of venous discirculation vary significantly from headache and nausea to pre-comatose state and death [14,17,22,26]. The variability of the clinical picture is determined by the number of segments of the venous system in which outflow disturbance occurs. This was shown in the experiment of Fries G et al. [14], when pigs were sequentially occluded from the superior sagittal sinus, pontine and cortical veins, while assessing the clinical condition of the animals, measuring ICP and water content in the brain tissue. It was found that severe cerebral edema, intracranial hypertension (ICH), destruction of the blood-brain barrier, and then arterial hypoperfusion leading to cerebral infarction developed only with simultaneous occlusion of the sinus, bridging and cortical veins, when both retrograde and collateral venous blood flow ceased [1, 14]. At the same time, it has been established that the most pronounced neurological deficit occurs when the outflow of blood to the deep and parasagittal veins is impaired or when a large number of the Venusylvian group is involved [24].
There are currently no generally accepted protocols for the correction of ICH that has developed as a result of impaired venous outflow. We present a clinical case of a patient with meningioma of the wing of the sphenoid bone, who in the early postoperative period acutely developed impaired venous outflow and persistent ICH.
Clinical observation.
Patient Ch., 47 years old, was admitted to the Institute with a diagnosis of “Tumor of the base of the middle cranial fossa on the left.” Upon admission, a decrease in visual acuity on the left was detected - counting fingers in the nasal part of the visual field, left-sided exophthalmos and signs of an effect on the left oculomotor nerve. An MRI examination of the brain revealed a meningioma at the base of the middle cranial fossa on the left (Figure 1).
An operation was performed - subtotal removal of meningioma of the medial parts of the base of the middle cranial fossa on the left using a pterional approach. The cavernous sinus was infiltrated by the tumor. This part of the tumor was not removed. Two large veins of the Sylvian group that had grown into the tumor capsule were coagulated intraoperatively.
Blood loss did not exceed 700 ml and was adequately replaced.
Awakening from anesthesia sleep was at the usual time. There was no increase in cerebral and focal neurological symptoms compared to the preoperative level.
The patient was extubated 2 hours after surgery. Hemodynamics were stable.
Homeostatic parameters were within normal limits.
The condition worsened sharply 12 hours after the operation. A coma and right-sided hemiparesis developed (3 – 4 points). Due to respiratory failure, the patient was intubated and mechanical ventilation was started in the SIMV+PS mode. A CT examination of the brain revealed predominantly left hemispheric edema, a massive focus of low density located in the fronto-parietal-temporal region on the left, and a displacement of the midline structures from left to right by 9 mm. The basal cisterns were not visualized, and the ventricular system was compressed (Fig. 2). Transcranial Doppler sonography showed linear blood flow velocity within normal limits. A subdural/parenchymal ICP sensor (Codman, USA) was installed. Severe ICH was detected (ICP 35 – 45 mmHg).
To correct ICH, options in the treatment protocol for patients with traumatic brain injury were used, since there are generally accepted protocols for correcting ICH when the venous drainage system is disrupted. The head end of the bed was raised 30º. Moderate hyperventilation, sedation with propofol (45 mcg/kg/min), analgesia with fentanyl (0.02 mcg/kg/min), and muscle relaxation with pipecuronium (0.5 mcg/kg/min) were started. These measures were ineffective - ICP remained at the level of 30 - 35 mmHg. 20 minutes after the start of therapy. After this, osmotherapy with mannitol (up to 1.5 g/kg) was used. After 10 minutes, ICP decreased to 20–25 mmHg, however, after 30 minutes, severe ICH developed again (up to 40 mmHg). Repeated mannitol infusion was ineffective.
A decision was made to proceed with moderate hypothermia. Induction of hypothermia began one hour after deterioration. External cooling and intravenous administration of cooled saline solution at a dose of 20 ml/kg were used. A temperature of 33º C was reached two hours after induction. ICP was effectively stabilized at 10–12 mmHg. During hypothermia, hypokalemia (3.1 – 3.3 mmol/l) and hypomagnesemia (0.39 – 0.41 mmol/l) developed, which were effectively corrected by the use of potassium and magnesium preparations. To prevent the development of hypocoagulation, taking into account the early postoperative period and the effects of hypothermia, plasma transfusion was performed at a dose of 15 ml/kg. At the same time, the prothrombin index was in the range of 75–85%, activated partial thromboplastin time was 28–33 seconds, fibrinogen was 3.4–3.9 g/l.
Body temperature was maintained at 33ºC for 24 hours. Before the start of warming, a control CT study of the brain was performed, which revealed positive dynamics in the form of the appearance of the basal cisterns and a decrease in the displacement of the median structures to the right to 5 mm. However, cerebral edema and a focus of low density in the left hemisphere persisted (Fig. 3).
Upon reaching 35ºC (10 hours after the start of rewarming), a clear tendency towards ICH appeared, so the rate of rewarming was reduced to ≈ 0.05 degrees per hour. Outside of sedation, the patient followed simple instructions, and the right-sided hemiparesis regressed.
Thus, already at this stage the positive effects of hypothermia were identified in the form of regression of general cerebral and focal neurological symptoms.
A temperature of 36ºC was reached after 20 hours. At this temperature, ICH developed up to 30 mmHg, resistant to sedation and osmotherapy with mannitol. This was an indication for performing external decompressive trepanation with plastic surgery of the dura mater. From the operation protocol it follows that, despite the tension of the dura mater, revealed after removal of the bone flap, the brain pulsated and was not vulnerable when touched. This was probably due to previous treatment, in particular the cerebroprotective effects of hypothermia. After dissection of the dura mater and expansion of the bone defect, the tension in the medulla decreased significantly, which made it possible to perform dura mater plastic surgery without technical difficulties. Decompression and plastic surgery of the dura mater made it possible to effectively stabilize ICP at a level of 10–15 mmHg.
Sedation continued for another 2 days after external decompression, and ICP monitoring continued for 5 days. There were no episodes of intracranial hypertension during this period. A CT examination revealed positive dynamics (Figure 4). The patient was conscious, but disoriented in place, time, and personal situation.
Movements were detected in all limbs, without clear asymmetry. Mechanical ventilation was stopped 6 days after decompressive trepanation, and after another 5 days the patient was transferred from intensive care to the neurosurgical department.
After 1.5 months, the patient’s condition was stable. Korsakov's syndrome was detected.
There were no speech or motor disturbances. An MRI study of the brain was performed with venography and in diffusion mode (Fig. 5), which revealed a lack of blood flow in the transverse and sigmoid sinuses on the left. In addition, an extensive lesion of cerebromalacia in the frontotemporal region of basal localization on the left was diagnosed, which was also visualized during a CT examination in the acute period as a focus of low density.
Analysis of clinical symptoms, CT and MRI data of the brain suggests that the cause of the formation of this lesion is an acute disturbance of venous outflow. In addition, an ischemic focus was visualized in the occipital region on the left, which has a cone-shaped shape and is located in the projection of the posterior cerebral artery basin. The most likely reason for the formation of this lesion is compression of the posterior cerebral artery on the tentorium of the cerebellum during swelling and dislocation of the brain in the acute period of the disease.
After 2.5 months, the patient developed hydrocephalus, which was an indication for lumboperitoneal shunting. After 4 months, the patient was discharged from the Institute in stable condition. The patient could care for herself independently.
The severity of Korsakoff's syndrome has decreased. 6 months after discharge, the patient is planned to be re-hospitalized to perform plastic surgery of the bone defect. The differential diagnosis for deterioration of the patient's condition was inter-arterial ischemia and impaired venous outflow. Venous disorders are characterized by: - absence of awakening from anesthesia sleep or deterioration of the condition several hours after surgery [17]; - CT examination can visualize a focus of reduced density in the first day after surgery due to hydrostatic edema [2]; - intraoperative data on damage to the veins or sinuses; - blood flow velocity during transcranial Doppler ultrasound remains normal or decreases [4,29,30,33]. - MRI venography reveals altered parameters [2]. However, performing an MRI examination in the acute period in the presence of severe ICH is fraught with the development of life-threatening complications.
So, differential diagnosis made it possible to suspect the presence of a venous outflow disorder in the patient. The pathogenesis of the developed condition can be represented as follows.
Intraoperative occlusion of the Sylvian veins included in the tumor led to local venous discirculation. The lack of blood flow through the transverse, sigmoid sinus (possibly congenital) and cavernous sinus (tumor infiltration) limited the compensatory possibilities of collateral redistribution of venous outflow. This led to a gradual increase in cerebral edema within 10–12 hours after surgery, which, in turn, caused discirculation in the system of deep veins of the brain.
This was the cause of acute deterioration 12 hours after surgery with the development of a comatose state due to severe cerebral edema and dislocation.
Currently, there is no protocol for correcting ICH in cases of impaired venous outflow.
The use of intensive care recommendations for the correction of ICH in patients with brain tumors, TBI and stroke was ineffective in our observation. Glucocorticosteroid hormones are not used for intensive treatment of cerebral edema caused by impaired venous outflow [17,26]. Raising the head of the bed, sedation, analgesia, muscle relaxation, moderate hyperventilation and osmotherapy were ineffective - ICH persisted.
If the listed options are ineffective in reducing ICP, the use of aggressive methods is indicated: barbiturate coma, external decompression or moderate hypothermia.
Barbiturates were not used because, according to the Cochrane Collaboration, which specializes in organizing and analyzing research results using the principles of evidence-based medicine, it follows that: “there is no evidence that barbiturate therapy in patients with severe traumatic brain injury improves outcomes. Barbiturates cause arterial hypotension in every fourth patient. The hypotensive effect of barbiturates will offset the positive effect of lowering ICP on cerebral perfusion pressure..." [28].
External decompressive trepanation was not used at this stage, since, according to Greenberg MS, when venous outflow is impaired due to venous thrombosis, it leads to a decrease in ICP, but does not improve disease outcomes [17]. With external decompression, intracranial relationships change, cerebrospinal fluid dynamics changes, and the risk of hemorrhage into the remnants of the tumor or the focus of ischemia, if present, increases.
It is known that with severe cerebral edema after decompression, entrapment of brain tissue in a bone defect can develop with the development of ischemia and secondary disruption of venous outflow in this area [11,27].
Moderate hypothermia (32 - 34ºС) is an effective method of controlling ICP [5,7,18], and has a cerebroprotective effect. The mechanism of cerebroprotection is to reduce the level of metabolism [6,9], reduce the permeability of the blood-brain barrier [13], reduce the concentration of excitatory amino acids and pro-inflammatory interleukins in damaged brain tissues [3,15], and reduce lipid peroxidation [19]. It is known that in experimental and clinical conditions, moderate hypothermia significantly reduces ICP, reduces the area of ischemic brain damage and can improve outcomes in a wide range of pathologies of the central nervous system: head injury, stroke, cerebral edema after cardiac arrest [10,16,20,23,25,31 ,32].
In the above observation, hypothermia during its implementation, firstly, effectively stabilized ICP, and secondly, it made it possible to protect the brain from ischemia. In contrast to oarterial ischemia, which develops, for example, due to clipping of an artery, when venous outflow is impaired, ischemia develops later. First, pronounced hydrostatic cerebral edema appears. This is precisely what manifested the deterioration of the condition in the above observation. If collateral or retrograde venous blood flow is not possible, then cerebral edema, incompatible with life, acutely develops. If collateral or retrograde venous blood flow is preserved, when its compensatory redistribution is possible, the severity of edema will vary significantly [14]. If the outflow disturbance persists, areas of the brain will form that will not receive an adequate volume of arterial blood. As a result, ischemia will develop. So, with venous infarction, hydrostatic edema first develops, and then ischemia and ischemic edema. In arterial infarction, ischemia develops first, followed by edema. Since in the above observation, when normothermia was achieved, there was regression of right-sided hemiparesis and restoration of consciousness, and subsequently there were no motor disorders, hypothermia probably had a cerebroprotective effect. In addition, the hypothermia provided adequate conditions for subsequent external decompression.
Hypothermia is an aggressive method for correcting ICH. The most frequently described complications in the literature are hypocoagulation [8,35], fluid and electrolyte disturbances [21], hemodynamic disorders [12,34] and infectious and inflammatory complications [8,34]. The most dangerous manifestations of hypocoagulation are intracranial hemorrhages. This led to the prophylactic use of fresh frozen plasma in the above observation. Fluid and electrolyte disturbances during hypothermia are manifested by hypokalemia and hypomagnesemia [21]. Hemodynamic disorders are usually manifested by sinus bradycardia. More dangerous rhythm disturbances have been described - asystole and ventricular fibrillation, however, they develop either at a temperature of less than 28ºC or with a duration of hypothermia of more than 48 hours [8,12,34]. The hypokalemia, hypomagnesemia and hemodynamically insignificant bradycardia that developed in the above observation did not threaten vital functions and were corrected immediately upon their development. Thus, moderate hypothermia has proven to be a relatively safe method of controlling ICP.
The warming period is an important stage in the management of hypothermia. In our observation, during warming, a tendency to increase ICP appeared. This is consistent with the data of a number of authors. Thus, Schwab S et al showed that warming is a period of high risk for the development of recurrent and persistent ICH, which can be fatal [31,32]. The authors proved that rewarming duration exceeding 16 hours significantly reduces mortality. Therefore, the rate of warming in our observation was ≈ 0.05º per hour. Despite this, stable ICH gradually developed, which became an indication for external decompressive trephination with dural plastic surgery. The chosen tactics made it possible to prevent increased brain dislocation and to preserve the structure of the brain parenchyma at the time of decompression.
Literature data and our observation indicate that hypothermia, due to its cerebroprotective effect, can reduce the area of an already formed ischemic focus and prevent further ischemic damage. However, during the warming period, the risk of developing recurrent resistant ICH remains. Under these conditions, it is necessary to perform external decompression with plastic surgery of the dura mater.
Hypothermia in this case will create more favorable conditions for performing the operation.
In conclusion, it must be said that in patients with tumors of basal localization in the early postoperative period, cerebral edema may develop due to impaired venous outflow. At the same time, stable ICH develops rapidly. It is quite difficult to differentiate impaired venous outflow from other possible causes of the development of cerebral edema in the acute period. However, this condition should always be considered as a possible cause for the development of persistent ICH. Moderate hypothermia is an effective and safe method for correcting cerebral edema and ICH in cases of impaired venous outflow. Intracranial hypertension that develops during the warming stage is an indication for external decompression. And, of course, further research is needed in this interesting and promising direction.
Literature
- Bekov D.B., Mikhailov S.S. “Atlas of arteries and veins of the human brain,” M. Medicine, 1979, pp. 95-96.
- Kornienko V.N., Pronin I.N. Diagnostic neuroradiology. M. Publishing house Andreeva T.M., 1368 pp. 2007.
- Aibiki M, Maekawa S, Ogura S, et al: Effects of moderate hypothermia on systemic and internal jugular plasma IL-6 levels after traumatic brain injury in humans. J Neurotrauma 1999; 16:225-232.
- Alexandrov A, Joseph M: Transcranial Doppler: An Overview of its Clinical Applications. The Internet Journal of Emergency and Intensive Care Medicine 2000; Vol. 4, No. 1 (ISSN: 1092–4051).
- Bayir H, Clark RSB, Kochanek PM Promising strategies to minimize secondary brain injury after head trauma. Crit Care Med 2003; 31:S112-S117.
- Berntman L, Welsh FA, Harp JR: Cerebral protective effect of low-grate hypothermia. Anesthesiology 1981; 55:495-498.
- Bernard SA, Buist M Induced hypothermia in critical care medicine: A review. Crit Care Med 2003; 31:2041–2051.
- Bernard S.A. Therapeutic hypothermia after cardiac arrest: Now a standard of care. Crit Care Med 2006; Vol. 34 P 923-924.
- Chopp M, Knight R, Tidwell CD, et al: The metabolic effects of mild hypothermia on global cerebral ischemia and recalculation in the cat: Comparison to normothermia and hyperthermia. J Cereb Blood Flow Metab 1989; 9:141-148.
- Colbourne F, Sutherland G, Gorbett D: Postischemic hypothermia: A critical appraisal with implications for clinical treatment. Mol Neurobiol 1997; 14:171-201.
- Csokay A, Pataki G, Nagy L, Belan K. Vascular tunnel construction in the treatment of severe brain swelling caused by trauma and SAH. (Evidence based on intra-operative blood flow measure). Neurological research 2002; 24:157-160.
- Danzl DF, Pozos RS: Accidental hypothermia. N Engl J Med 1994; 331:1756–1760.
- Dietrich WD, Busto R, Halley M, et al: The importance of brain temperature in alterations of the blood-brain barrier following cerebral ischemia. J Neuropathol Exp Neurol 1990; 49:486-497.
- Fries G, Wallenfang T, Hennen J, et al Occlusion of pig superior sagittal sinus, bridging and cortical veins: multistep evolution of sinus-vein thrombosis. J. Neurosurg. 1992; 77:127-133.
- Globus MY, Alonso O, Dietrich WD, et al: Glutamate release and free radical production following brain injury: Effects of posttraumatic hypothermia. J Neurochem 1995; 65:1704–1711.
- Goto Y, Kassell NF, Hiramatsu K, et al: Effects of intraischemic hypothermia on cerebral damage in a model of reversible focal ischemia. Neurosurgery 1993; 32:980-984.
- Greenberg MS. Handbook of neurosurgery. pp 609,611,891. Thieme, 2001.
- Hofmeijer J, van der Worp H, Kappelle LJ Treatment of space-occurring cerebral infarction. Crit Care Med 2003; 31:617-625.
- Karibe H, Chen SF, Zarow GJ, et al: Mild intraischemic hypothermia suppress consumption of endogenous antioxidants after temporary focal ischemia in rats. Brain Res 1994; 649:12-18.
- Kawai N, Okauchi M, Morisaki K, et al: Effects of delayed intraischemic and postischemic on a focal model of transient cerebral ischemia in rats. Strike 2000; 31:232-239.
- Koht A, Cane R, Cerullo LJ: Serum potassium levels during prolonged hypothermia. Int Care Med 1983; 9:275 – 277.
- Kurita H, Shin M, Ueki K, Kawamoto S, Kirino T. Congestive brain oedema associated with a pial arteriovenous malformation with impaired venous drainage. Acta Neurochir. 2001; 143:339-342.
- Maier CM, Sun GH, Kunis D, et al: Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: Neurological outcome and infarct size. J Neurosurg. 2001; 94:90-96.
- McElveen WA, Gonzales RF, Keegan AP: Cerebral venous thrombosis. E-medicine (https://www.emedicine.com/neuro/topic642.htm), 2006.
- Nolan JP, Morley PT, Hoek TL, et al: Therapeutic hypothermia after cardiac arrest. An advisory statement by the Advancement Life supporting Task Force of the International Liaison Committee on Resuscitation. Resuscitation 2003; 57:231-235.
- Park JH, Yoon SH New concept of cerebrospinal fluid dynamics in cerebral venous sinus thombosis. Medical Hypotheses 2007, article in press.
- Polin RS, Shaffrey ME, Bogaev CA. Decompressive bifrontal craniectomy in the treatment of severe post-traumatic cerebral edema. Neurosurgery 1997; 41:84-92.
- Roberts I. Barbiturates for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2005(2):CD000033.
- Saqqur M, Zygun D, Demchuk A: Role of transcranial Doppler in neurocritical care. Crit Care Med 2007; 35(5) Suppl May: S216-S223.
- Schreiber SJ, Stolz E, Valdueza JM: Transcranial ultrasonography of cerebral veins and sinuses. Eur J Ultrasound 2002 Nov; 16(1-2): 59-72.
- Schwab S, Georgiadis D, Berrouschot J, et al: Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke 2001; 32:2033–2035.
- Schwab S, Schwarz S, Spranger M, et al: Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke 1998; 29:2461–2466.
- Stolz E, Kaps M, Kern A, et al.: Transcranial color-coded duplex sonography of intracranial veins and sinuses in adults. Reference data from 130 volunteers. Stroke 1999 May;30(5): 1070 – 1075.
- The hypothermia after cardiac arrest study group: mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. New Engl J Med 2002; 346:549-556.
- Valeri CR, MacGregor H, Cassidy G, et al: Effects of temperature on bleeding time and clotting time in normal male and female volunteers. Crit Care Med 1995; 23:698-704.
Literature
- Arac A., Blanchard V., Steinberg GK Assessment of outcome following decompressive craniectomy for malignant middle cerebral artery infarction in patients older than 60 years of age. Neurosurg. Focus. 2009; 26:E3. DOI: 10.3171/2009.3.FOCUS0958
- Bohman LE, Schuster JM Decompressive craniectomy for management of traumatic brain injury: an update. Curr. Neurol. Neurosci. Rep. 2013; 13: 392. DOI: 10.1007/s11910-013-0392-x
- Bor-Seng-Shu E., Figueiredo EG, Amorium RL, et al. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain surgery. J. Neurosurg. 2012; 117:589–596. DOI: 10.3171/2012.6.JNS101400
- Coutinho JM Cerebral venous thrombosis. J. Thromb. Haemost. 2015: 13(Suppl. 1): S238–S244. DOI: 10.1111/jth.12945
- Merenda A., DeGeorgia M. Craniectomy for acute ischemic stroke: how to apply the data to the bedside. Curr. Opin. Neurol. 2010; 23:53–58. DOI: 10.1097/WCO.0b013e328334bdf4
- Rahme R., Zuccarello M., Kleindorfer D., et al. Decompressive hemicraniectomy for malignant middle cerebral artery territory infarction: is life worth living? J. Neurosurg. 2012; 117:749–754. DOI: 10.3171/2012.6.JNS111140
- Raza E., Shamim MS, Wadiwala MF, et al. Decompressive surgery for malignant cerebral venous sinus thrombosis: a retrospective case series from Pakistan and comparative literature review. J. Stroke Cerebrovasc. Dis. 2014; 23:e13–e23. DOI: 10.1016/ j.jstrokecerebrovasdis.2013.07.045
- Sahuquillo J., Martinez-Ricarte F., Poca MA Decompressive craniectomy in traumatic brain injury after DECRA trial. Where do we stand? Curr. Opin. Crit. Care. 2013; 19: 101–106. DOI: 10.1097/MCC.0b013e3285eba1a
- Wijdicks EF, Sheth KN, Carter BS, et al. AHA Stroke Council. Recommendations for management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45: 1222–1238. DOI: 10.1161/01.str.0000441965.15164.d6
- Hill CS, Luoma AM, Wilson SR, Kitchen N. Titanium cranioplasty and prediction of complications. Br. J. Neurosurg. 2012; 26:832–837. DOI: 10.3109/02688697.2012.692839
- Klinger DR, Madden C, Beshay J, et al. Autologous and acrylic cranioplasty: a review of 10 years and 258 cases. World Neurosurg. 2014; 82:e525–e530. DOI: 10.1016/j.wneu.2013.08.005
- Schwarz F., Dunisch P., Walter J., et al. Cranioplasty after decompressive craniectomy: is there a rationale for an initial artificial bone-substitute implant? A single-center experience after 631 procedures. J. Neurosurg. 2015; 124:1–6. DOI: 10.3171/2015.4.JNS159
- Durga P., Sahu BP Neurological deterioration during intubation in cervical spine disorders. Indian J. Anaesth. 2014; 58:684–692. DOI: 10.4103/0019–5049.147132
- Harris EA Airway management for the patient with an unstable cervical spine. In: Ruskin KJ, Rosenbaum SH, Rampil IJ (Eds.). Fundamentals of Neuroanesthesia. 2014. Oxford Univ. Press. P. 288–303.
- Amoozegar F., Ronskley PE, Sauve R., Menon BK Hormonal contraceptives and cerebral venous thrombosis risk: a systematic review and meta-analysis. Front. Neurol. 2015; 6, A7: 1–11. DOI: 10.3389/fneur.2015.00007
- Coutinho JM, Majoie CB, Coert BA, Stam J. Decompressive hemicraniectomy in cerebral sinus thrombosis: consecutive case series and review of the literature. Stroke. 2009; 40:2233–2235. DOI: 10.1161/STROKEAHA.108.543421
- Ferro JM, Canhao P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr. Radiol Rep. 2014; 16: 523. DOI: 10.1007/s11886-014-0523-2
- Fischer C., Goldstein J., Edlow J. Cerebral venous sinus thrombosis in the emergency department: retrospective analysis of 17 cases and review of the literature. J. Emerg. Med. 2010; 38: 140–147. DOI: 10.1016./jemermed.2009.08.061
- Kenmur CL, Jovin T., Jadhav A. Cerebral venous sinus thrombosis in users of a hormonal vaginal ring. Obstet. Gynecol. 2015; 126:830–833. DOI: 10/1097/AOG.0000000000000931
- Siudut J., Swiat M., Undas A. Altered fibrin clot properties in patients with cerebral venous sinus thrombosis: Association with the risk of recurrence. Stroke. 2015; 46:2666–2668. DOI: 10.1161/STROKEAHA.115.009528
- Agner C., Dujovny M., Gaviria M. Neurocognitive assessment before and after cranioplasty. Acta Neurochir. 2002; 144:1033–1040.
- Decaminada N., Pernter P., Imondi A., Tomassini A. CT perfusion evaluation of cerebral hemodynamics before and after cranioplasty. Neuroradiol. J. 2008; 21: 459–471. DOI: 10.1177/19714009080210042
- Erdogan E., Duz B., Kokaoglu M., Izci Y., Nimurkaynak E. The effect of cranioplasty on cerebral hemo-dynamics: evaluation with transcranial Doppler sonography. Neurol. India. 2003; 51:479–481.
- Kemmling A., Duning T., Lemcke L., et al. Case report of MR perfusion imaging in sinking skin flap syndrome: growing evidence for hemodynamic impairment. BMC Neurol. 2010; 10: 80. DOI: 10.1186/1471-2377-10-80
- Jelcic N., Della Puppa A., Mottaran R., et al. Case series evidence for improvement of executive functions after late cranioplasty. Brain Inj. 2013; 27: 1723–1726. DOI: 10.3109/02699052.2013.844857.
- Jeyaraj P. Importance of early cranioplasty in reversing the “Syndrome of the trephine/motor trephine syndrome/sinking skin flap syndrome.” J. Maxillofac. Oral. Surg. 2015; 14:666–673. DOI: 10.1007/s12663-014-0673-1
- Kuo JR, Wang CC, Chio CC, Cheng TJ Neurological improvement after cranioplasty - analysis by transcranial Doppler ultrasonography. J. Clin. Neurosci. 2004; 11:486–489. DOI: 10.1016/j.jocn.2003.06.005
- Song J., Liu M., Mo X., et al. Beneficial impact of early cranioplasty in patients with decompressive craniectomy: evidence from trenscranial Doppler ultrasonography. Acta Neurochir. 2014; 156:193–198. DOI: 10.1007/s00701-013-1908-5
- Winkler PA, Stummer W, Linke R, et al. The influence of cranioplasty on postural blood flow regulation, carabrovascular reserve capacity, and cerebral glucose metabolism. Neurosurg. Focus. 2000; 8: e9. DOI: 10.3171/foc.2000.8.1.1920
- Sakamoto S., Eguchi K., Kiura Y., et al. CT perfusion imaging in the syndrome of the sinking flap before and after cranioplasty. Clin. Neurol. Neurosurg. 2006; 108:583–585. DOI: 10.1016/j.clineuro.2005.03.012
- Annan M., De Toffol B., Hommet C., Mondon K. Sinking skin flap syndrome (or syndrome of the trephined): A review. Br. J. Neurosurg. 2015; 29: 314–318. DOI: 10.3109/02688697.2015.1012047
- Honeybul S. Neurological susceptibility to a skull defect. Surg. Neurol. Int. 2014; 5: 83. DOI: 10.4103/2152–7806.133886
- Honeybul S., Janzen C., Kruger K., Ho KM The incidence of neurological instability to a skull defect. World Neurosurg. 2015; pii: S1878–8750(15)01249–8. DOI: 10.1016/j.2neu.2015.09.081.
- Chan KW, Datta NN Iatrogenic acute subdural hematoma due to drainage catheter. Surg. Neurol. 2000; 54:444–446.
- Karamchandani K., Chouhan RS, Bithal PK, Dash HH Severe bradicardia and hypotension after connecting negative pressure to the subgaleal drain during craniotomy closure. Br. J. Anaesth. 2006; 96:608–610. DOI: 10.1093/bja/ael063
- Mohindra S., Mukherjee KK, Chhabra KK, Khosla VK Subgaleal suction drain leading to fatal sagittal sinus hemorrhage. Br. J. Neurosurg. 2005; 19: 352–354. DOI: 10.1080/02688690500305308
- Prabhakar H., Bithal PK, Chouhan RS, Dash HH Rupture of intracranial aneurysm after partial clipping due to aspiration drainage system—a case report. Middle East J. Anaesthesiol. 2008; 19: 1185–1190.
- Roth J., Galeano E., Milla S., et al. Multiple epidural hematomas and hemodynamic collapse caused by a subgaleal drain and suction-induced intracranial hypotension: case report. Neurosurgery. 2011; 68:E271–E276. DOI: 10.1227/NEU.0b013e3181fe6165
- Toshniwal GR, Bhagat H., Rath GP Bradycardia following negative pressure suction of subgaleal drain during craniotomy closure. Acta Neurochir. 2007; 149:1077–1079. DOI: 10.1007/s00701-007-1246-6
- Van Roost D., Thees C., Brenke C., et al. Pseudohypoxic brain swelling: a newly defined complication after uneventful brain surgery, probably related to suction drainage. Neurosurgery. 2003; 53:1315–1326. DOI: 10.1227/01.neu.0000093498.08913.9e
- Yadav M., Nikhar SA, Kulkarni DK, Gopinath R. Cardiac arrest after connecting negative pressure to the subgaleal drain during craniotomy closure. Case Rep Anesthesiol. 2014; 2014: Article ID 146870. DOI: 10.1155/2014/146870
- Chitale R., Tjoumakaris S., Gonzalez F., et al. Infratentorial and supratentorial strokes after cranioplasty. Neurologist. 2013; 19: 17–21. DOI: 10.1097/NRL.0b013e31827c6bb6
- Honeybul S. Sudden death following cranioplasty: a complication of decompressive craniectomy for head injury. Br. J. Neurosurg. 2011; 25: 343–345. DOI: 10.3109/02688697.2011.568643
- Lee GS, Park SQ, Kim R., Cho SJ Unexpected severe cerebral edema after cranioplasty: Case report and literature review. J. Korean Neurosurg. Soc. 2015; 58: 76–78. DOI: 10.3340/jkns.2015.58.1.76
- Santana-Cabrera L., Perez-Ortiz C., Rodriguez-Escort C., Sanchez-Palacios M. Massive postoperative swelling following cranioplasty. Int. J. Crit. Illn. Inj. 2012; 2: 107–108. DOI: 10.4103/2229–5151.97277
- Zebian B., Critchley G. Sudden death following cranioplasty: a complication of decompressive craniectomy for head injury. Br. J. Neurosurg. 2011; 25: 785–786. DOI: 10.3109/02688697.2011.623801
- Robles A., Cuevas-Solorzano A. Massive brain swelling and death after cranioplasty: A systematic review. World Neurosurg. 2018; 111:98–108. DOI: 10.1016/wneu2017.12.061
- Spetzler RF, Wilson CD, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin. Neurosurg. 1978; 25: 651–672.
- Petrozza PH Hyperemic complications following resection of arterio-venous malformation: New througts. J. N. S. Anesthet. 1995; 7:202.
- Dodson BA Normal perfusion pressure breakthrough syndrome: Entity or excuse? J. N. S. Anesthet. 1995; 7:203–207.
- Al-Rodhan NRF Occlusive hyperemia remains the most logical explanation for the hemodynamic complications of resected intracerebral arteriovenous malformations. J. N. S. Anesthet. 1995; 7:208–210.
- Moulakakis KG, Mylonas SN, Styroeras GS, Andrikopoulos V. Hyperperfusion syndrome after carotid revascularization. J.Vasc. Surg. 2009; 49:1060–1068. DOI: 10.1016/j.jvs.2008.11.026
- Medel R., Crowley RW, Dumont AS Hyperperfusion syndrome following endovascular cerebral revascularization. A review. Neurosurg. Forum. 2009; 26:E4. DOI: 10.3171.2009.1.FOCUS08276
- Lieb M., Shah U., Hines GL Cerebral hyperperfusion syndrome after carotid intervention: A review. Cardiol. Rev. 2012; 20: 84–89. DOI: 10.1097/CRD.0b013e318237eef8
- Farooq MU, Goushganian C., Min J., Gorelik PB Pathophysiology and management of reperfusion injury and hyperperfusion syndrome after carotid endarterectomy and carotid artery stenting. Exp. Transl. Stroke Med. 2016; 8: 7. DOI: 10.1186/s13231-016-0021-2
Recovery after craniotomy
The first postoperative day is decisive for the patient. He is transferred to the intensive care ward, where vital processes are supported by special medical devices.
The medical staff vigilantly monitors the patient's awakening after anesthesia.
If necessary, the surgical wound is drained, ensuring the removal of excess fluid. The cleanliness of the drainage requires careful care, since infection can lead to meningitis.
Craniotomy is a serious operation and the stitches are removed after 7-10 days.
The length of stay in intensive care is at least a week and depends solely on the speed of the patient’s recovery abilities.
Drug treatment during the rehabilitation period is aimed at preventing the development of complications or the emergence of new pathologies.
- Painkillers are required. Patients are concerned about both real and phantom pain, including psychosomatic pain.
- Antibiotics help prevent inflammation.
- Antiemetics and anticonvulsants prevent the most common brain injury syndromes.
- Diuretics are used to prevent brain swelling.
The bandage is changed daily, and after two days the patient tries to get up. With a good recovery rate, after a few days the patient can move around independently quite confidently.
Recovery is not limited to the hospital. After returning home, the patient must also carefully follow all medical instructions. Strictly prohibited:
- lifting weights more than 3 kg;
- bends;
- smoking and alcohol;
- stress.
Even if there are no obvious errors in pronunciation, regular sessions with a speech therapist are recommended to eliminate speech disorders.
Daily short, accompanied walks and a balanced, low-salt diet are helpful.
If the emotional background of a patient prone to depression cannot be corrected, you need to consult a psychologist.