| Primary motor cortex | |
| Brodmann area 4 of the human brain. | |
| The primary motor cortex is shown in green. | |
| Details | |
| Part | Precentral gyrus |
| Artery | Forebrain Midbrain |
| Identifiers | |
| Latin | cerebral cortex motor primus |
| NeuroNames | 1910 |
| NeuroLex ID | nlx_143555 |
| F.M.A. | 224854 |
| Anatomical terms of neuroanatomy [edit in Wikidata] | |
Animation.
The primary motor cortex (Brodmann area 4) of the left cerebral hemisphere is shown in red. Primary motor cortex
(Brodmann area 4) is a region of the brain that in humans is located in the dorsal part of the frontal lobe. It is a core area in the musculoskeletal system and works in conjunction with other motor areas, including the premotor cortex, the supplementary motor area, the posterior parietal cortex, and several subcortical areas of the brain, to plan and execute movements. The primary motor cortex is anatomically defined as the area of the cortex containing large neurons known as Betz cells. Betz cells, along with other cortical neurons, send long axons down the spinal cord to synapse with interneuronal circuits of the spinal cord, as well as directly with alpha motor neurons in the spinal cord that connect to muscles.
In the primary motor cortex, motor representation is ordered (inverted) from the toe (at the top of the cerebral hemisphere) to the mouth (at the bottom) along a fold in the cortex called the central sulcus. However, some parts of the body may be controlled by partially overlapping areas of the cortex. Each cerebral hemisphere of the primary motor cortex contains only the motor representation of the opposite (contralateral) side of the body. The amount of primary motor cortex dedicated to a body part is not proportional to the absolute size of the body surface, but instead to the relative density of cutaneous motor receptors on said body part. The density of cutaneous motor receptors on a body part usually indicates the necessary degree of precision movement required for that body part. For this reason, human hands and face have a much larger image than feet.
For the discovery of the primary motor cortex and its relationship with other motor cortical areas, see the main article on motor cortex.
CONTENT
- 1 Structure 1.1 Path
- 1.2 Corticomotor neurons
- 1.3 Blood supply
- 2.1 Homunculus
Structure [edit]
The human primary motor cortex is located on the anterior wall of the central sulcus. It also extends partially anteriorly from the sulcus onto the precentral gyrus. Anteriorly, the primary motor cortex is bounded by a set of areas that lie on the precentral gyrus and which are generally considered to constitute the lateral premotor cortex. Posteriorly, the primary motor cortex is bounded by the primary somatosensory cortex, which lies on the posterior wall of the central sulcus. Ventrally, the primary motor cortex is limited to the insular cortex in the lateral sulcus. The primary motor cortex extends dorsally to the top of the hemisphere and then continues to the medial wall of the hemisphere.
The location of the primary motor cortex is most obvious on histological examination due to the presence of characteristic Betz cells. Layer V of the primary motor cortex contains giant (70-100 µm) pyramidal neurons, which are Betz cells. These neurons send long axons to the contralateral motor nuclei of the cranial nerves and to the lower motor neurons of the ventral horn of the spinal cord. These axons are part of the corticospinal tract. Betz cells make up only a small percentage of the corticospinal tract. By some estimates, they constitute about 10% of the primary motor cortex neurons projecting to the spinal cord [1], or about 2–3% of the total cortical projection to the spinal cord. [2] Although Betz cells do not account for all of the motor cortex's output, they are nonetheless a clear marker for the primary motor cortex. This area of the cortex, characterized by the presence of Betz cells, was named area 4 by Brodmann.
Path[edit]
As the primary motor axons pass down through the white matter of the brain, they come closer together and form part of the posterior
parts of the inner capsule.
They continue down into the brain stem, where some of them, moving to the contralateral side, spread to the motor nuclei of the cranial nerves. ( Note
: several motor fiber synapses with lower motor neurons on the same side of the brainstem).
After crossing to the contralateral side in the medulla oblongata (pyramidal decussation), the axons move along the spinal cord along the lateral corticospinal tract
.
Fibers that do not cross in the brainstem travel along a separate ventral corticospinal tract, and most of them pass to the contralateral side of the spinal cord shortly before reaching the lower motor neurons. In addition to the main corticospinal tract, the motor cortex projects to other cortical and subcortical areas, including the striatum, hypothalamus, midbrain and hindbrain, as well as the basal ganglia of the thalamus, midbrain and medulla [3].
Corticomotor neurons [edit]
Corticomotor neurons
- These are neurons of the primary cortex that project directly to the motor neurons of the ventral horn of the spinal cord. [4] [5] The axons of corticomotor neurons terminate on spinal motor neurons of several muscles, as well as on spinal interneurons. [4] [5] They are unique to primates, and their function has been proposed to be adaptive control of distal limbs (such as the hands), including relatively independent control of individual digits. [5] Corticomotor neurons have so far only been found in the primary motor cortex, but not in the secondary motor areas. [5]
Blood supply [edit]
Branches of the middle cerebral artery provide most of the arterial blood supply to the primary motor cortex.
The medial side (leg areas) is supplied by branches of the anterior cerebral artery.
Subregions [edit]
At least six areas are now recognized within the larger region once defined as the SMA. These divisions have been studied most extensively in the monkey brain. The most anterior portion is now commonly referred to as the pre-SMA. [1][2][3] It has sparse or no connections with the spinal cord or primary motor cortex, and also has extensive connections with prefrontal regions. [1] [4] [5] [6] [7]
The extra eye field (SEF) is the relatively anterior part of the SMA that, when stimulated, causes movements of the head and eyes and possibly movements of the limbs and torso. [8] [9] [10] [11]
Dahm and Strick [5] hypothesized, based on cytoarchitecture and connections to the spinal cord, that the portion of the SMA in the cingulate sulcus in the medial hemisphere may be divided into three distinct regions, the cingulate motor areas. The functions of the motor areas of the cingulate cortex have not been systematically studied.
The SMA proper in monkeys is now limited to the area at the vertex of the hemisphere and extends partly to the medial wall, just before the presentation of the primary motor leg. The SMA itself projects directly to the spinal cord and is therefore one of the main output areas of the cortical motor system. [5] [12] [13] [14] [15] [16]
Recently, Zhang et al. [17] examined the functional subdivisions of medial SFC based on whole-brain connectivity characterized from a large resting-state fMRI dataset. In addition to reproducing the boundaries between the SMA and preSMA, the current results support a functional difference between the posterior and anterior pre-SMA. In contrast to the posterior pre-SMA, the anterior pre-SMA is associated with most prefrontal but not somatomotor areas. In general, the SMA is strongly connected to the thalamus and epithalamus, the posterior pre-SMA to the putamen, pallidum and STN, and the anterior pre-SMA to the caudate nucleus, with the caudate nucleus showing significant hemispheric asymmetry.
Function [edit]
Homunculus [edit]
The various parts of the body in the primary motor cortex are widely represented in a structure called the motor homunculus (Latin: little man
). [6] The leg region is located close to the midline, in the inner parts of the motor area, which passes into the medial longitudinal fissure. The lateral, convex side of the primary motor cortex is located from top to bottom in areas that correspond to the buttocks, torso, shoulder, elbow, wrist, fingers, thumbs, eyelids, lips and jaw. The motor area of the arm and hand is the largest and occupies part of the precentral gyrus between the leg and the face area.
These areas are not proportional to their size on the body, with the lips, parts of the face and hands represented in particularly large areas. After amputation or paralysis, motor areas may shift to accommodate new parts of the body.
Neural signal from the thalamus[edit]
The primary motor cortex receives thalamic inputs from various thalamic nuclei. Among other things:
— Ventral-lateral nucleus of cerebellar afferents
— Ventral anterior nucleus of basal ganglia afferents
Alternative maps[edit]
Map of the body in the human brain
At least two modifications of the classical somatotopic ordering of body parts have been reported in primate primary motor cortex.
First, the hand representation can be organized into a stem and an environment. In the monkey cortex, the fingers of the hand are represented in a central region at the posterior edge of the primary motor cortex. This central region is surrounded on three sides (dorsally, anteriorly, and ventrally) by more proximal portions of the arm, including the elbow and shoulder. [7] [8] In humans, the image of the fingers is surrounded dorsally, anteriorly, and below by the image of the wrist. [9]
A second modification of the classical somatotopic ordering of body parts is the dual representation of fingers and wrist, studied mainly in the human motor cortex. One representation is in a posterior region called area 4p, and the other is in an anterior region called area 4a. The posterior region can be activated by attention without any sensory feedback and has been suggested to be important for the initiation of movements, while the anterior region is dependent on sensory feedback. [10] It can also be activated by imaginary finger movements [11] and listening to speech without making actual movements. This anterior representational area is hypothesized to be important for the execution of movements involving complex sensorimotor interactions. [12] It is possible that area 4a in humans corresponds to some parts of the caudal premotor cortex, as described in the monkey cortex.
In 2009, it was reported that there are two evolutionarily distinct regions: an older one on the outer surface and a new one found in the crevice. The older one connects to the motor neurons of the spinal cord through spinal cord interneurons. The newer one, found only in apes and apes, connects directly to motor neurons in the spinal cord. [13] Direct connections are formed after birth, predominate over indirect connections, and are more flexible in the patterns they can develop, allowing postnatal learning of complex fine motor skills. "Thus, the emergence of a 'new' M1 region during the evolution of the primate lineage was likely important for increased manual dexterity in humans." [14]
Common misconceptions[edit]
Certain misconceptions about the primary motor cortex are common in secondary reviews, textbooks, and popular materials. Here are three of the most common misconceptions.
Segregated body map[edit]
One of the most common misconceptions about the primary motor cortex is that the body map is neatly divided. However, this is not a map of individual muscles or even individual body parts. The map has significant overlap. This overlap increases in more anterior areas of the primary motor cortex. One of the major goals in the history of motor cortex research has been to determine the extent to which different body parts overlap or are segregated within the motor cortex. Researchers who have looked into this issue have found that the map of the hand, arm, and shoulder overlaps greatly. [6] [8] [9] [15] [16] [17] [18] [19] Studies that map the precise functional connectivity between cortical neurons and muscles show that even a single neuron in the primary motor cortex can influence activity of many muscles associated with many joints. [15] In experiments with cats and monkeys, as the animals learn complex, coordinated movements, the map in the primary motor cortex becomes increasingly overlapping, apparently learning to integrate control over many muscles. [20] [21] In monkeys, when electrical stimulation is applied to the motor cortex on a behavioral time scale, it produces complex, highly integrated movements, such as reaching with a hand shaped to grasp, or bringing a hand to the mouth and opening the mouth. [22] [23] This type of evidence suggests that the primary motor cortex, although containing a rough map of the body, may be involved in integrating muscles in a meaningful way rather than dividing control over individual muscle groups. It has been suggested that the deeper organizing principle may be a map of statistical correlations in the behavioral repertoire rather than a map of body parts. [23] [24] To the extent that the movement repertoire is partially broken down into the actions of individual body parts, the map contains a coarse and overlapping arrangement of the body.
M1 and primary motor cortex[edit]
The term "M1" and the term "primary motor cortex" are often used interchangeably. However, they come from different historical traditions and belong to different parts of the cerebral cortex. Some scientists have proposed that the motor cortex may be divided into the primary motor stripe, which is more posterior, and the lateral premotor stripe, which is more anterior. Early researchers who originally proposed this view included Campbell, [25] Vogt and Vogt, [26] Förster, [27] and Fulton. [28] Others have suggested that the motor cortex cannot be divided in this way. Instead, in this second image, the so-called primary motor and lateral premotor striae together make up a single cortical area called M1. The second motor area on the medial wall of the hemisphere has been called M2 or supplementary motor area. Proponents of this point of view were Penfield [6] and Wolsey. [29] A distinction is now generally accepted between the primary motor cortex and the lateral premotor cortex. However, the term M1 is sometimes mistakenly used to refer to the primary motor cortex. Strictly speaking, M1 refers to a single map that, according to some previous researchers, encompassed both the primary motor and lateral premotor cortices.
Betz cells as the last common pathway[edit]
The Betz cells, or giant pyramidal cells in the primary motor cortex, are sometimes mistakenly thought to be the only or main exit from the cerebral cortex to the spinal cord. This is an old mistake, dating back at least to Campbell in 1905. [25] However, Betz cells constitute only about 2–3% of neurons that project from the cortex to the spinal cord, [2] and only about 10% of neurons are neurons that project from the primary motor cortex to the spinal cord. [1] A number of cortical areas, including the premotor cortex, supplementary motor area, and even the primary somatosensory cortex, project to the spinal cord. Even when Betz cells are damaged, the cortex can still communicate with subcortical motor structures and control movement. If the primary motor cortex with its Betz cells is damaged, temporary paralysis results, and other areas of the cortex can apparently take over some of the lost function.
ZONES OF THE LARGER HEMISPHERES
Localization of functions in the cerebral hemispheres. The cerebral cortex is divided into main zones, consisting of several cortical fields. Each of these zones performs a certain general function, and its constituent fields are specialized in the implementation of individual elements of this function. However, thanks to the conductive pathways, several zones of the cerebral hemispheres, certain subcortical centers, nuclei of the brain stem and segments of the spinal cord are involved in the implementation of individual links of higher and lower nervous activity.
With the subtle and precise specialization of certain groups of neurons, the brain and spinal cord function as a single unit. Mental functions of the brain are also not limited to individual areas of the cortex, but are the result of the joint activity of large areas of the cerebral hemispheres and subcortical centers.
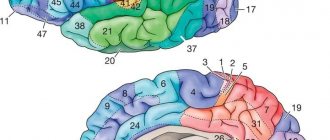
Rice. 123. Individual changes in the main fields of the neocortex of the cerebral hemispheres in three adults (A, B, C). Numbers are fields according to Brodmann. The motor zone (field 4) is located in the anterior central gyrus along the central sulcus. In the upper quarter of the zone there are motor centers for the leg muscles.
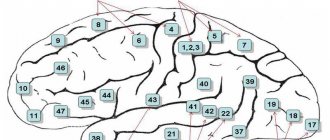
At the top are the neurons that innervate the muscles of the toes, and at the bottom - the thighs and torso. The middle two quarters are occupied by the centers for the arms, above - the center of the scapula muscles, and below - the muscles of the fingers. And finally, in the lower quarter of the anterior central gyrus there are centers of the muscles of the face and speech apparatus. As a result of the historical development of the human brain in the process of work and speech, a particularly large place is occupied by groups of neurons that cause contraction of the muscles of the hand, mainly the thumb, and the muscles of the face, tongue and larynx. They receive centripetal fibers from proprioceptors, entering along the dorsal roots into the spinal cord, where they rise as part of the posterior column of the same side to the nuclei of the tender and wedge-shaped fasciculi of the medulla oblongata. From these nuclei the fibers of the second neurons emerge, forming a medial loop and, after decussation, reaching the nuclei of the optic thalamus of the opposite side. From here, most of the centripetal fibers of the third neurons reach the posterior central gyrus and then enter the anterior central gyrus, and a smaller part enters it directly. Thus, the anterior central gyrus is connected to the posterior central gyrus through fibers passing through the pathways of the cortex. From the motor zone emerge centrifugal motor fibers of pyramidal neurons, which make up the pyramidal pathways; they reach the neurons of the anterior horns of the spinal cord. The motor area causes coordinated movements of skeletal muscles, mainly on the opposite side of the body. It functions together with the subcortical centers - the striatal bodies, as well as the Lewis body, the red nucleus and the substantia nigra. When certain areas of the anterior central gyrus are damaged, voluntary movements of individual muscle groups are impaired. Incomplete damage to the zone causes movement disorders—paresis, and its complete destruction—paralysis. The area of musculocutaneous sensitivity (fields 1, 2, 3, 43 and partially 5 and 7) is located in the posterior central gyrus along the posterior central sulcus. In this zone, the granular layers of the cortex are especially strongly developed, to which centripetal fibers from skin receptors approach, running as part of the same pathways as fibers from proprioceptors. The location of the perceptive groups of neurons is the same as in the motor zone. The largest surface area is occupied by neurons that receive impulses from the receptors of the hand, face, tongue and larynx. Field 7 is more associated with hand sensitivity than other fields. The zone of musculocutaneous sensitivity is not completely delimited from the motor zone, since in fields 3, 4 and 5 there is a combination of granular neurons with giant pyramidal neurons. The motor zone contains approximately 80% of motor neurons, and the musculocutaneous sensitivity zone contains 20%. Each hemisphere receives impulses mainly from receptors on the opposite side of the body, but also from receptors on the same side. Centripetal impulses enter this zone mainly from the lateral and semilunar nuclei of the thalamus optic. With lesions of certain areas of the posterior central gyrus, sensitivity in certain areas of the skin is impaired. The loss of the ability to recognize objects by touch is referred to as tactile agnosia. When the functions of the zone are impaired, disorders of touch, pain and temperature sensations of the skin and muscle-joint sensitivity are observed. Incomplete damage to the zone causes a decrease in reception - hyposthesia, and complete damage - its loss - anesthesia. The frontal zone (fields 6, 5, 9, 10, 11, 44, 45, 46, 47) is located in the frontal lobe in front of the motor lobe. It is divided into premotor and speech motor. The premotor zone (fields 6, 8, 9, 10, 11) regulates the tone of skeletal muscles and coordinated movements of the body, orienting it in space. Field 46 is functionally connected with field 10, which is involved in the execution of motor conditioned reflexes. Centripetal impulses from the internal organs enter the premotor zone and a significant part of the centrifugal vegetative fibers emanate from it. Therefore, damage to the premotor zone causes impaired coordination of movements - ataxia and dysfunction of the cardiovascular, respiratory, digestive and other systems of internal organs. The visual zone (fields 17, 18, 19) is located on the inner surface of the occipital lobe on both sides of the calcarine groove. In humans, it occupies 12% of the total surface of the cortex. Area 17 is located at the occipital pole; it is surrounded by area 18, which surrounds area 19, which borders the posterior limbic region, superior and inferior parietal regions. In field 17, the central field of the visual zone, there are 16 times more neurons than in the central field of the auditory zone (field 41), and 10 times more neurons than in the central field of the motor zone (field 4). This indicates the leading importance of vision in the historical and individual development of man. From the retina, 900 thousand - 1 million centripetal fibers of the optic nerves reach the external geniculate body, in which individual parts of the retina are accurately projected. The centripetal fibers of the neurons of the lateral geniculate body are directed to the visual zone, mainly to the main visual field 17. Other intermediate visual centers involved in the transmission of not visual impulses, but oculomotor ones, are the cushion of the visual thalamus and the anterior tubercles of the quadrigeminal. Before entering the external geniculate body, the fibers of the optic nerve intersect. Thanks to this decussation, the visual pathway heading to the visual zone of each hemisphere contains 50% of the fibers on its side and 50% of the fibers on the opposite side. The visual zone of the left hemisphere receives visual impulses from the left halves of the retinas of both eyes, and the zone of the right hemisphere receives from the right halves of the retinas of both eyes. Therefore, the destruction of one of the visual zones causes blindness in the same halves of the retinas in both eyes - hemianopsia. In the optic nerves, in addition to centripetal fibers, there are also somewhat thicker centrifugal fibers to the muscles of the iris and centrifugal thin sympathetic fibers from the neurons of the subcortical centers. A small part of the centripetal fibers of the optic nerve is not interrupted in the subcortical formations, but is directly sent to the cerebellum and visual areas of the cerebral hemispheres. The destruction of both fields 17 causes complete cortical blindness, the destruction of field 18 leads to loss of visual memory while maintaining vision, which is designated as visual agnosia, and the destruction of field 19 leads to loss of orientation in an unusual environment. The auditory zone (fields 41, 42, 21, 22, 20, 37) is located on the surface of the temporal lobe, mainly the anterior transverse temporal gyrus and the superior temporal gyrus. Area 41, located in the superior temporal gyrus and in the anterior part of the transverse gyrus, is a projection of the organ of Corti of the cochlea. From the organ of Corti, centripetal impulses pass through the spiral ganglion along the cochlear nerve, consisting of approximately 30 thousand fibers. This node contains the first bipolar neurons of the auditory pathway. Next, the fibers of the first neurons transmit auditory impulses to the nuclei of the auditory nerve in the medulla oblongata, where the second neurons are located. The fibers of the auditory nerve nuclei communicate with the nuclei of the facial nerve in the medulla oblongata and the oculomotor nerve in the anterior tubercles of the midbrain. Therefore, when strong sounds occur, the muscles of the face, eyelids, and auricle reflexively contract and eye movements are caused. Most of the fibers of the auditory nerve nuclei decussate in the pons, and the smaller part passes on its side. Then the fibers of the auditory pathway enter the lateral lemniscus, which ends in the posterior tuberosity of the quadrigeminal and in the internal geniculate body, where the third neurons are located - their fibers conduct centripetal impulses to the auditory zone. There are also direct pathways connecting the nuclei of the auditory nerves with the cerebellum and auditory area. Most of the direct cerebellar tracts are formed by the vestibular nerve, and a smaller part by the cochlear nerve, which together make up the common trunk of the auditory nerve. The vestibular apparatus is also projected in the auditory zone. Destruction of field 41 on one side causes deafness on the opposite side and weakened hearing on one’s side, and destruction of field 41 on both sides leads to complete cortical deafness. Destruction of field 22 in the anterior third of the superior temporal gyrus leads to musical deafness - the perception of the intensity of tone, timbre and rhythm of sounds is lost - auditory agnosia. Destruction of areas 21 and 20 in the middle and inferior temporal gyri causes ataxia, a disorder of balance and coordination of movements. The speech-auditory center is also located in the auditory zone. Olfactory and gustatory zones. The olfactory zone is located in the ancient cortex, which receives centripetal impulses from the olfactory cells. In addition to the olfactory function, it also performs a gustatory function and is involved in the activities of the digestive, excretory and reproductive systems. Previously, it was believed that the hippocampus has an olfactory function. It is currently believed that, together with the limbic system, the hypothalamic region of the diencephalon and pituitary gland, the midbrain and medulla oblongata, and especially the reticular formation, the hippocampus is involved in general motor reactions and autonomic reflexes during emotions. The taste zone itself is probably located in area 43, which is located in the lower part of the posterior central gyrus.
The limbic gyrus (posterior area 23 and anterior area 24) and the insular cortex (areas 13 and 14) are involved in higher nervous activity. All zones of the cortex are not isolated, but are interconnected by conducting pathways. Speech centers (fields 44, 45, 46, 39, 40, 42, 22,37). The motor speech center is located in the lower part of the anterior central gyrus in area 44. In most right-handed people, the area of area 44 in the left hemisphere is larger than in the right hemisphere. Field 44 causes the complex contractions of the speech muscles necessary to pronounce words. When this field is destroyed, a person cannot speak, but can produce the simplest contractions of the speech muscles - screaming and singing. This is a motor aphasia, which in some cases manifests itself in the absence of contractions of the muscles of the tongue and other speech muscles. Since in these cases the auditory center of speech is not damaged, the understanding of the speech of others is preserved. When field 44 is damaged, not only oral speech is often impaired, but also internal speech or the ability to formulate thoughts in words without pronouncing them, based on accumulated sound images that have a certain semantic content. At the same time, reading to oneself is difficult, the ability to write freely and under dictation is impaired, but copying letters when writing is preserved. In right-handed people, motor aphasia is observed when the left hemisphere is damaged, and in left-handed people, when the right hemisphere is damaged.
Rice. 129. Localization of speech centers: 1 - motor, 2 - auditory, 3 - visual. In front of field 44 there is field 45, which regulates the construction of grammatically correct combinations of words and singing. When this field is damaged due to loss of memory for pronunciation techniques, singing becomes upset. Facial expressions and gestures, which give speech its expressiveness, are carried out thanks to impulses coming from field 46 to fields 44 and 45, to the fields of the premotor area and to the subcortical centers. The auditory, or sensory, center of speech is located in the posterior part of the left superior temporal gyrus in area 42, which carries out the understanding of a word when hearing it. If the field is destroyed, the ability to understand the meaning of words is lost, but their perception as sounds is preserved - sensory aphasia, or speech deafness. At the same time, due to a lack of understanding of one’s own speech, excessive talkativeness is sometimes observed - logorrhea, or verbal diarrhea. In the rear part of field 22, connections between sound images of words and all perceptual zones in which ideas about objects and phenomena arise are recorded. Therefore, damage to this field also causes sensory aphasia. Fields 39 and 40, located in the parietal lobe next to field 22, carry out understanding of the meaning of combinations of words or phrases. Therefore, their defeat leads to a speech disorder called semantic aphasia. If field 39 is affected, due to the loss of the ability to recognize letters and numbers and understand the meaning of visible written images of words and numbers, the ability to read aloud, write and count is lost. A lesion of field 40 causes loss of the ability to write, since there is no orientation of movements in space and their sequence is disrupted. This lack of ability to produce systematic, purposeful movements (apraxia) does not exclude the ability to correctly perform individual hand movements not related to writing. Consequently, the process of writing in right-handers is carried out by the temporal, inferior parietal and inferior frontal regions of the left hemisphere. When field 37 is damaged, memory loss for words is caused - amnestic aphasia. Thus, the cerebral hemispheres as a whole are involved in the implementation of the speech function, but a special role is played by individual fields of the cortex. In right-handed people, as a result of the preferential development of the functions of the right hand and the right half of the body, the most complex mental functions of the left hemisphere of the brain are especially developed.
Related materials:
Brain phylogeny
The structure of the cerebral hemispheres
Blood supply to the brain
Cerebrospinal fluid
Motion coding[edit]
Evarts [30] proposed that each neuron in the motor cortex contributes to the force in the muscle. When a neuron becomes active, it sends a signal to the spinal cord, the signal is transmitted to the motor neuron, the motor neuron sends a signal to the muscle, and the muscle contracts. The higher the activity of the motor cortex neuron, the greater the muscle strength.
Georgopoulos and colleagues [31] [32] [33] suggested that muscle strength alone is too simple a description. They trained monkeys to reach different directions and monitored the activity of neurons in the motor cortex. They found that each motor cortex neuron was maximally active in a particular reach direction and less responsive to adjacent reach directions. On this basis, they proposed that motor cortex neurons, by "voting" or combining their influences into a "population code", could accurately determine the direction of a reach.
The proposal that motor cortex neurons encode reach direction has become controversial. Scott and Kalaska [34] showed that each neuron in the motor cortex correlates better with details of joint movement and muscle force than with reach direction. Schwartz and colleagues [35] showed that motor cortex neurons correlate well with hand speed. Strick and colleagues [36] found that some neurons in the motor cortex are active in relation to muscle force and some in relation to the spatial direction of movement. Todorov [37] proposed that the many different correlations are the result of a muscle regulator in which many movement parameters are correlated with muscle strength.
The code by which primate motor cortex neurons control the spinal cord and therefore movement remains controversial.
Some progress in understanding how the motor cortex produces movement has also been made in rodent models. The rodent motor cortex, like the monkey motor cortex, may contain subregions that emphasize different general types of actions. [38] [39] For example, one area seems to emphasize the rhythmic control of whisking. [38] [40] [41] Neurons in this area project to a specific subcortical nucleus, in which a pattern generator coordinates the cyclic rhythm of the whiskers. This nucleus then projects to the muscles that control the whiskers.
Links[edit]
- ^ ab Rivara CB, Sherwood CC, Bouras C, Hof PR (2003). "Stereological characterization and spatial distribution patterns of Betz cells in the human primary motor cortex". Anatomical Recording, Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology
.
270
(2):137–151. DOI: 10.1002/ar.a.10015. PMID 12524689. - ^ ab Lassek AM (1941). "The pyramidal tract of the monkey." J. Comp. Neurol
.
74
(2):193–202. DOI: 10.1002/cne.900740202. S2CID 83536088. - "View Sections". brainarchitecture.org
. Retrieved November 20, 2021. - ^ ab Siegelbaum, Stephen A.; Hudspeth, A. J. (2013). Principles of Neuroscience
. Kandel, Eric R. (5th ed.). NY. ISBN 9780071390118. OCLC 795553723. - ^ abcd Limon, Roger N. (April 4, 2008). "Descending pathways in motor control". Annual Review of Neuroscience
.
31
(1):195–218. DOI: 10.1146/annurev.neuro.31.060407.125547. ISSN 0147-006X. PMID 18558853. - ^ abc Penfield W. and Baldry E. (1937). "Somatic motor and sensory representations in the human cerebral cortex when studied using electrical stimulation". Brain
.
60
(4):389–443. DOI: 10.1093/brain/60.4.389.CS1 maint: multiple names: authors list (link) - Jump up
↑ Kwan HC, MacKay WA, Murphy JT, Wong YC (1978).
“Spatial organization of the precentral cortex in awake primates. II. Motor outputs." J. Neurophysiol
.
41
(5):1120–1131. DOI: 10.1152/jn.1978.41.5.1120. PMID 100584. - ^ ab Park, M.K., Belhadj-Saif, A., Gordon, M. and Chaney, P.D. (2001). "Consistent features in forelimb representation of primary motor cortex in rhesus monkeys". J. Neurosci
.
21
(8):2784–2792. DOI: 10.1523/JNEUROSCI.21-08-02784.2001. PMID 11306630. CS1 maint: multiple names: list of authors (link) - ^ ab Meyer, J. D., Aflalo, T. N., Kastner, S., & Graziano, M. S. A. (2008). "The complex organization of the human primary motor cortex: a high-resolution fMRI study". J. Neurophysiol
.
100
(4):1800–1812. DOI: 10.1152/jn.90531.2008. PMC 2576195. PMID 18684903. CS1 maint: multiple names: list of authors (link) - Binkofski F, Fink GR, Geyer S, Buccino G, Gruber O, NJ Shah, Taylor JG, Seitz RJ, Zilles K, Freund HJ (2002). "Neural activity in areas 4a and 4b of human primary motor cortex is differentially modulated by attention to action." J. Neurophysiol
.
88
(1):514–519. DOI: 10.1152/jn.2002.88.1.514. PMID 12091573. - Nikhil Sharma; PS Jones; T. A. Carpenter; Jean-Claude Baron (2008). "Mapping BA 4a and 4p participation during Motor Imagery." NeuroImage
.
41
(1):92–99. DOI: 10.1016/j.neuroimage.2008.02.009. PMID 18358742. S2CID 8673179. - Terumitsu M, Ikeda K, Kwee IL, & Nakada, T (2009). "Involvement of area 4a of primary motor cortex in complex sensory processing: a 3.0T fMRI study." NeuroReport
.
20
(7): 679–683. DOI: 10.1097/WNR.0b013e32832a1820. PMID 19339906. S2CID 23674509. CS1 maint: multiple names: list of authors (link) - Rathelot, J.-A.; Strik, Poland (20 January 2009). "Divisions of the primary motor cortex based on corticomotoneuronal cells". Proc.
Natl. Academician Sci .
106
(3):918–923. Bibcode: 2009PNAS..106..918R. DOI: 10.1073/pnas.0808362106. PMC 2621250. PMID 19139417. - Costandi, Mo (2009). "The Evolution of Sleight of Hand". Proceedings of the National Academy of Sciences
.
106
(3):918–923. DOI: 10.1073/pnas.0808362106. PMC 2621250. PMID 19139417. Retrieved November 29, 2015. - ^ ab Cheney, P. D. & Fetz, E. E. (1985). "Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and individual intracortical microstimuli in primates: evidence for functional groups of CM cells." J. Neurophysiol
.
53
(3):786–804. DOI: 10.1152/jn.1985.53.3.786. PMID 2984354. - Schieber, M. H. & Hibbard, L. S. (1993). “How somatotopic is the area of the motor cortex of the hand?” The science
.
261
(5120):489–492. Bibcode: 1993Sci…261..489S. DOI: 10.1126/science.8332915. PMID 8332915. - Rathelot, J. A. & Strick, P. L. (2006). "Muscle representation in the macaque motor cortex: an anatomical perspective". Proc.
Natl. Academician Sci. USA .
103
(21):8257–8262. Bibcode: 2006PNAS..103.8257R. DOI: 10.1073/pnas.0602933103. PMC 1461407. PMID 16702556. - Sanes, Y. N., Donogh, D. P., Thangaraj, V., Edelman, R. R., & Warach, S. (1995). "Shared neural substrates controlling hand movements in human motor cortex". The science
.
268
(5218): 1775–1777. Bibcode: 1995Sci…268.1775S. DOI: 10.1126/science.7792606. PMID 7792606. CS1 maint: multiple names: list of authors (link) - Donogh, DP, Leibovic, S. and Sanes, YN. (1992). "Organization of the forelimb area in the motor cortex of the squirrel monkey: representation of the muscles of the fingers, wrists, and elbows." Exp.
Brain Res .
89
(1): 1–10. DOI: 10.1007/bf00228996. PMID 1601087. S2CID 1398462. CS1 maint: multiple names: list of authors (link) - Nudo, R. J., Milliken, G. W., Jenkins, W. M., & Merzenich, M. M. (1996). "Use-dependent changes in motion representations in the primary motor cortex of adult squirrel monkeys". J. Neurosci
.
16
(2):785–807. DOI: 10.1523/JNEUROSCI.16-02-00785.1996. PMC 6578638. PMID 8551360. CS1 maint: multiple names: list of authors (link) - Martin, J. H., Engber, D., & Meng, Z. (2005). "The influence of forelimb use on the postnatal development of forelimb motor representation in the primary motor cortex of the cat." J. Neurophysiol
.
93
(5):2822–2831. DOI: 10.1152/jn.01060.2004. PMID 15574795. CS1 maint: multiple names: list of authors (link) - Jump up
↑ Graziano, M. S. A., Taylor, C. S. R. and Moore, T. (2002).
"Complex movements induced by microstimulation of the precentral cortex". Neuron
.
34
(5):841–851. DOI: 10.1016/S0896-6273(02)00698-0. PMID 12062029. S2CID 3069873. CS1 maint: multiple names: list of authors (link) - ^ a b Graziano, M. S. A. (2008). Intelligent motion machine
. Oxford, UK: Oxford University Press. - Graziano, M. S. A., & Eflalo, T. N. (2007). "Mapping the behavioral repertoire to the cortex". Neuron
.
56
(2):239–251. DOI: 10.1016/j.neuron.2007.09.013. PMID 17964243. CS1 maint: multiple names: list of authors (link) - ^ ab Campbell, A. W. (1905). Histological studies on the localization of cerebral function. Cambridge, MA: Cambridge University Press.
- Jump up
↑ Vogt, C. and Vogt, O. (1919).
"Ergebnisse unserer Hirnforschung". Journal für Psychologie und Neurologie
.
25
: 277–462.CS1 maint: several names: list of authors (link) - Jump up
↑ Foerster, O (1936).
"The Human Motor Cortex in the Light of the Doctrines of Hughlings Jackson". Brain
.
59
(2): 135–159. DOI: 10.1093/brain/59.2.135. - Jump up
↑ Fulton, J (1935).
"A note on the definition of 'motor' and 'premotor' areas." Brain
.
58
(2):311–316. DOI: 10.1093/brain/58.2.311. - ↑
Woolsey, C. N., Settlage, P. H., Meyer, D. R., Sencer, W., Hamuy, T. P. and Travis, A. M. (1952).
"Localization patterns in premotor areas and their relation to the concept of the premotor area". Association for Research in Nervous and Mental Diseases
.
New York, NY: Raven Press. 30
: 238–264.CS1 maint: several names: list of authors (link) - Evarts, E. W. (1968). "Relationship of pyramidal tract activity with force applied during voluntary movement." J. Neurophysiol
.
31
(1): 14–27. DOI: 10.1152/jn.1968.31.1.14. PMID 4966614. - Georgopoulos, A. P., Kalaska, J. F., Caminiti, R. Massey, J. T. (1982). "On the relationship between the direction of two-dimensional hand movements and cell discharge in primate motor cortex". J. Neurosci
.
2
(11): 1527–1537. DOI: 10.1523/JNEUROSCI.02-11-01527.1982. PMC 6564361. PMID 7143039. CS1 maint: multiple names: list of authors (link) - Georgopoulos, A. P., Kettner, R. E., & Schwartz, A. B. (1988). “Primate motor cortex and free-hand movements toward visual targets in three-dimensional space. II. Coding of movement direction by a neuronal population". J. Neurosci
.
8
(8):2928–2937. DOI: 10.1523/JNEUROSCI.08-08-02928.1988. PMC 6569382. PMID 3411362. CS1 maint: multiple names: list of authors (link) - Georgopoulos, A. P., Schwartz, A. B., & Kettner, R. E. (1986). "Neural population coding of movement direction". The science
.
233
(4771):1416–1419. Bibcode: 1986Sci...233.1416G. DOI: 10.1126/science.3749885. PMID 3749885. CS1 maint: multiple names: list of authors (link) - Scott, S. H. & Kalaska, J. F. (1995). "Changes in motor cortex activity during movements to achieve a similar arm trajectory, but with different arm positions." J. Neurophysiol
.
73
(6):2563–2567. DOI: 10.1152/jn.1995.73.6.2563. PMID 7666162. - Jump up
↑ Moran, D. W. & Schwartz, A. B. (1999).
"Motor cortical representation of speed and direction in reaching". J. Neurophysiol
.
82
(5):2676–2692. DOI: 10.1152/jn.1999.82.5.2676. PMID 10561437. - Kakei, S., Hoffman, D., & Strick P (1999). "Muscle and movement representations in the primary motor cortex". The science
.
285
(5436):2136–2139. CiteSeerX 10.1.1.137.8610. DOI: 10.1126/science.285.5436.2136. PMID 10497133. CS1 maint: multiple names: list of authors (link) - Jump up
↑ Todorov, E (2000).
"Direct cortical control of muscle activation during voluntary arm movements: a model". Nat Neurosci
.
3
(4): 391–398. DOI: 10.1038/73964. PMID 10725930. S2CID 13996279. - ^ ab Haiss, F. & Schwarz, C (2005). "Spatial segregation of different motor control modes in whisker representations in rat primary motor cortex". J. Neurosci
.
25
(6):1579–1587. DOI: 10.1523/JNEUROSCI.3760-04.2005. PMC 6726007. PMID 15703412. - Ramanathan, D., Conner, J. M., & Tuszynski, M. H. (2006). "A form of motor cortical plasticity that correlates with functional recovery after traumatic brain injury". Proc.
Natl. Academician Sci. USA .
103
(30):11370–11375. Bibcode: 2006PNAS..10311370R. DOI: 10.1073/pnas.0601065103. PMC 1544093. PMID 16837575. CS1 maint: multiple names: list of authors (link) - Brecht, M., Schneider, M., Sakmann, B., & Margrie, T. W. (2004). "Whisker movements evoked by stimulation of single pyramidal cells in the rat motor cortex". Nature
.
427
(6976):704–710. Bibcode: 2004Natur.427..704B. DOI: 10.1038/nature02266. PMID 14973477. S2CID 1105868. CS1 maint: multiple names: list of authors (link) - Jump up
↑ Cramer, N. P. & Keller, A. (2006).
"Cortical control of the central churning pattern generator". J. Neurophysiol
.
96
(1):209–217. DOI: 10.1152/jn.00071.2006. PMC 1764853. PMID 16641387.
Further reading[edit]
- Principles of Neuroscience (2000), 4th ed., Kandel et al.
- Debaer, F., Wenderoth, N., Sunaert, S., Van-Heck, P., Swinnen, S.P. (July 2003). "Internal and external movement generation: differential neural pathways involved in bimanual coordination performed in the presence and absence of enhanced visual feedback." NeuroImage
.
19
(3): 764–76. DOI: 10.1016/s1053-8119(03)00148-4. PMID 12880805 .CS1 maint: multiple names: authors list (link) - Vorobiev; and others. (1998). "Partial division of human mesial region 6: cytoarchitectonic evidence for three distinct regions". Eur J Neurosci
.
10
(6):2199–203. DOI: 10.1046/j.1460-9568.1998.00236.x. PMID 9753106.