General information about focal epilepsy
The definition of “focal epilepsy” combines all forms of epileptic paroxysms that arise due to the presence of a local focus of increased epi-activity in cerebral structures.
Epileptic activity begins focally, but can spread from the focus of excitation to the surrounding brain tissue, which causes secondary generalization of the seizure. It is important to differentiate paroxysms of FE and attacks of generalized epilepsy with a primary diffuse nature of excitation. In addition, there is a multifocal form of epilepsy. In this form of epilepsy, there are several local epileptogenic zones in the brain. Approximately 82% of all epileptic syndromes are focal epilepsy, and in 75% of cases it begins in childhood. Most often it occurs against the background of traumatic, ischemic or infectious damage, or disorders of brain development. Secondary focal epilepsy of this nature is diagnosed in 71% of all patients suffering from epilepsy.
What can trigger a seizure in a patient with epilepsy?
Seizures usually occur without antecedent factors (randomly) and are completely unpredictable. However, some patients notice certain conditions that may trigger attacks. After identifying provoking factors, measures can be taken to avoid them, which will help reduce the frequency of epileptic seizures in the future. Examples of factors that trigger attacks in some patients include flashing lights, sleep restriction, stressful situations, and the use of alcohol or certain medications.
Women with epilepsy often report an increase in the frequency of seizures during menstruation, which is likely due to hormonal changes and fluid retention.
Pathogenesis of focal epilepsy
The causes of the development of focal epilepsy are: developmental defects that affect a limited area of the brain (cerebral arteriovenous malformations, focal cortical dysplasia, congenital cerebral cysts, etc.), traumatic brain injuries, infections (brain abscess, encephalitis, neurosyphilis, cysticercosis), vascular system disorders (previous hemorrhagic stroke), metabolic encephalopathy, brain tumors. In PE, one of the etiological factors with preserved morphology of neurons and the medulla as a whole may be acquired and genetically determined metabolic defects of neurons in a certain zone of the cerebral cortex.
Perinatal lesions of the central nervous system are the leading cause among the factors causing focal epilepsy. Such lesions are: fetal hypoxia, asphyxia of the newborn, intracranial birth injury, intrauterine infections. The appearance of a focal pathological focus in childhood may be associated with impaired cortical maturation.
The pathophysiological basis of FE is the epileptogenic focus, in which several zones are distinguished. The zone of pathological damage corresponds to the area of morphological changes in cerebral tissue, which can be recorded using MRI.
The primary zone is the part of the cerebral cortex in which epi discharges are generated.
The symptomatogenic zone is the area of the cortex, when excited, an epileptic attack occurs. The irritative zone is an area that is recorded on the EEG during the interictal interval and is the source of an epileptic seizure.
The zone of functional deficit is the area responsible for the neurological disorders that accompany epileptic seizures.
Are epileptic seizures life-threatening for the patient?
Epilepsy is often thought to be a benign disease with low mortality. However, in fact, epilepsy is associated with an increased mortality rate, especially for young patients (under 40 years of age) and patients with severe epilepsy. In general, the mortality rate in patients with epilepsy is 1.5–3 times higher than in the general population (according to population studies). Mortality rates are higher in men than in women and higher in the first 10 years after epilepsy diagnosis.
The most common causes of death in people with epilepsy are infectious diseases of the chest organs (bronchopneumonia, especially in the elderly), neoplasms and deaths directly related to seizures. Death extremely rarely occurs as a result of side effects of treatment (acute and severe drug intolerance reactions).
In part, the increased risk for patients with epilepsy is due to the fact that epilepsy may be based on severe or progressive brain disease (tumors, trauma, cerebrovascular disease, etc.). And it is these diseases, and not attacks as such, that can cause a poor prognosis.
On the other hand, repeated epileptic seizures can create many situations that threaten the patient's life. Deaths directly related to seizures fall into several categories: status epilepticus, seizure-related deaths, sudden death syndrome, and accidents.
The most dangerous status epilepticus is generalized convulsive seizures. Death occurs in 10% of all cases of generalized tonic-clonic seizures.
Classification of focal epilepsy
There are symptomatic, idiopathic and cryptogenic forms of focal epilepsy. In the symptomatic form, it is possible to establish the source and cause of the pathology, as well as identify morphological changes that are recorded during tomographic studies.
A feature of cryptogenic PE is that, despite its secondary nature, none of the existing imaging methods is capable of identifying morphological abnormalities in the structure of the brain.
Idiopathic PE is not characterized by brain defects typical for this group of pathologies. The substrate for its development, as a rule, is a hereditary predisposition: genetically determined channelopathies, defects in the membranes of CNS cells, dysgenesis of the cerebral cortex. The prognosis for this disease is favorable, the course is benign. Idiopathic FE includes: benign rolandic epilepsy, childhood occipital Gastaut epilepsy, Panagiotopoulos syndrome, benign occipital epileptic syndromes.
Hereditary epilepsy and genes
In 70–80% of cases, this disease is genetically determined. To date, more than 400 genes are known, mutations in which can cause the disease. Parents can be carriers of such a mutation and are able to pass it on to their children.
Febrile seizures are the most common form of seizure syndrome. This disease is mapped on chromosomes 8ql3-21, 2q23-24, 19p13.3. Genes predisposing to juvenile myoclonic hereditary epilepsy are on chromosomes 6p 11-12 and 15ql4, to adult - on chromosome 8q24, to infant - in chromosomal locus 16p13.
The study of genetic markers allows us to determine the form of this disease and the likelihood of its inheritance. You can take such a test at the medical genetics department. The “Hereditary Epilepsy” panel provides information that helps plan treatment tactics for the disease, predict its course, exclude the need for surgery and make decisions regarding childbearing.
Symptoms of focal epilepsy
Focal epilepsy is characterized by partial focal epileptic paroxysms. Paroxysms can be simple, not accompanied by loss of consciousness, and complex, without loss of consciousness. Simple partial epileptic seizures can be vegetative, somatosensory, motor, sensory, with a hallucinatory component and with mental disorders.
Complex partial epileptic seizures may begin as simple ones, but then damage consciousness occurs. There may be confusion after a seizure.
Secondarily generalized partial seizures begin as a simple or complex focal seizure, but then the excitation spreads to other parts of the cerebral cortex. Thus, the paroxysm takes on a clonic-tonic appearance. The patient may experience various types of partial paroxysms.
Symptomatic focal epilepsy is accompanied by symptoms that correspond to the underlying brain lesion. This type of epilepsy is characterized by decreased intelligence, delayed mental development and impairment of the child’s cognitive sphere.
Idiopathic focal epilepsy is benign and is not characterized by disorders of the mental and intellectual spheres.
Epilepsy in children and adolescents is the most important socio-medical problem of our time. Despite the wide range of available antiepileptic drugs (AEDs), seizures continue in more than 25% of children with epilepsy [30]. Traditional AEDs are not always effective enough [56]. Therefore, a large number of works have now appeared in the literature on the therapeutic effectiveness of new AEDs [36].
In the treatment of focal forms of epilepsy, AEDs of different generations are used. In 1912, the first anticonvulsant drug phenobarbital was synthesized, which remained the only AED on the pharmaceutical market for more than 30 years. Since the 60s, it has been the first and second choice drug for various types of epileptic seizures. However, its use in children is currently limited due to its pronounced sedative effect [50]. Nevertheless, phenobarbital still occupies a certain place in the treatment of children with epilepsy, especially at an early age [24]. Phenobarbital is used for all forms of epilepsy accompanied by generalized seizures [35]. It is more effective for simple partial seizures and less effective for complex partial seizures. It should not be prescribed for absence seizures, myoclonic-astatic seizures and West syndrome. Phenobarbital is often prescribed for neonatal seizures [19, 21, 29], with the exception of use in children born weighing less than 1800 grams [65], as well as for typical febrile seizures [46, 51]. It is a second-line drug for the relief of status epilepticus [43, 60]. Valproate and carbamazepine are also widely used in the treatment of epilepsy in children.
In the treatment of focal forms of epilepsy in childhood, new generation AEDs are often used - topiramate (Topamax, Janssen-Silag) and lamotrigine (Lamictal, GlaxoSmithKlain). It should be noted that these drugs have appeared on the domestic pharmaceutical market since the 90s and are used in pediatric practice when traditional AEDs are ineffective and, as a rule, in combination with them [48].
Topiramate (Topamax) is a new highly effective drug with a wide therapeutic spectrum [17, 41]. It belongs to the class of sulfamate-substituted monosaccharides and has a complex mechanism of action [18, 37]. Topiramate is indicated for partial and generalized seizures, both in monotherapy and in combination with other AEDs [39]. It is used in the treatment of seizures associated with Lennox-Gastaut syndrome [38, 40] and West syndrome [14, 57], and can be prescribed to adults and children with newly diagnosed epilepsy.
Lamotrigine (Lamictal) is a broad-spectrum drug for the treatment of all types of seizures [10, 12, 26], with the exception of myoclonic ones [22]. It is recommended for focal and generalized, idiopathic and symptomatic epileptic syndromes in adults and children [59]. Lamotrigine prevents the effect of secondary seizure generalization by inhibiting the diffuse spread of epileptiform activity [10]. It is also the drug of choice in the treatment of Lennox-Gastaut syndrome [42], West syndrome [13], and Angelman syndrome [25].
The purpose of this study was to analyze the effectiveness of AEDs of different pharmacological groups in the treatment of focal forms of epilepsy in children in a city epileptology center.
Material and methods
The study included 96 patients, 55 (57%) boys and 41 (43%) girls, with a verified diagnosis: symptomatic focal epilepsy or presumably symptomatic (cryptogenic) focal epilepsy. The age of the patients varied from 1 month to 17 years. Patients were observed from the age of 6 months to 4 years 6 months.
The diagnosis of epilepsy and determination of the nature of seizures were carried out in accordance with the International Classification of Epilepsy, Epileptic Syndromes and Related Diseases (New Delhi, 1989).
In each case, a detailed collection of anamnestic data was carried out and the neurological status was assessed.
EEG was recorded over time (in some cases, video-EEG monitoring was performed including the sleep period). The study was carried out according to the standard 10-20 method on a 19-channel Neuron-spectrum device (Ivanovo) or an Encephalan-131-03 device, modification 11, (Taganrog). Neuroimaging included computed tomography of the brain or MRI on 0.5-1.5 Tesla tomographs. General and biochemical blood tests were also monitored to exclude side effects of therapy.
The main reasons for including patients in the study were the lack or loss of effect from previous therapy and the persistence of attacks, as well as the occurrence of undesirable side effects when using traditional AEDs; in isolated cases, new AEDs were used in initial monotherapy.
According to the recommendations of the International League Against Epilepsy (1998), in recent years restrictions have been introduced on the use of phenobarbital in childhood due to possible side effects on the development of cognitive functions in children. Therefore, the source of information about patients receiving phenobarbital was the medical records of inpatients treated for epilepsy in a neurological clinic in 2000-2005. They made up the 1st group of patients. This group included 34 patients aged from 1 month to 11 years 9 months. In 94% of cases, phenobarbital was used as initial monotherapy. Subsequently, 18% of patients received combination therapy with phenobarbital and other AEDs. Doses of the drug in the study ranged from 12 to 300 mg per day, with an average of about 170 mg per day. Per kilogram of body weight, doses of phenobarbital were prescribed in wide therapeutic ranges from 1.5 to 12 mg/kg per day, averaging 6.4 mg/kg per day. The 2nd group of patients consisted of 31 patients aged from 24 months to 16 years and received topiramate therapy. In 12 (39%) patients, the drug was used as starting or alternative monotherapy. Topiramate was prescribed in combination with one AED to 13 (42%) patients. Combination therapy of topiramate with 2 AEDs was performed in 6 (19%) patients. Doses of the drug in the study ranged from 56 to 500 mg per day, averaging about 165 mg per day. Per kilogram of body weight, doses of topiramate were prescribed in wide therapeutic ranges from 2.8 to 17 mg/kg per day, with an average of 6.6 mg/kg per day.
Group 3 patients were treated with lamotrigine. It was used in 31 patients ranging in age from 1 year 10 months to 17 years. In all cases, the drug was prescribed as additional therapy to one or two other AEDs. During treatment, 3 (10%) patients were switched to alternative monotherapy with this drug. Lamotrigine was prescribed in doses ranging from 25 to 250 mg per day, with an average of 116 mg per day. Based on the amount of drug per child's body weight, doses varied in the range from 0.5 to 6 mg/kg per day, averaging 3.6 mg/kg per day. In all three groups, drug titration was carried out according to generally accepted recommendations for drug administration.
The general characteristics of patients in each group are given in Table. 1.
The clinical criterion for the effectiveness of therapy is the cessation of seizures or a change in their frequency during antiepileptic therapy. Therefore, the result was assessed by the effect on the frequency of attacks according to standard criteria: complete clinical remission (100% relief of attacks for 12 months or more); reduction in the frequency of attacks by 50% or more; reduction in the frequency of attacks by less than 50% or no effect; aggravation of attacks. The impact of the studied AEDs after their introduction on the quality of attacks was assessed: their nature, duration and intensity.
results
In each group, the majority were children with symptomatic focal forms of epilepsy - 77, 85 and 86% of cases. Accordingly, the proportion of patients with cryptogenic focal forms of epilepsy was significantly smaller - 23, 15 and 14%, respectively. In all three groups, symptomatic focal frontal lobe epilepsy was more often detected - 20, 26, 39%; multifocal epilepsy - 12, 19, 22.5%. Since before 2005 there was no technical ability to conduct EEG monitoring, in the group of patients treated with phenobarbital, a non-localized form of epilepsy was diagnosed in 35% of cases.
Analyzing and comparing the history of the development of the disease, it was revealed that epilepsy most often debuted before the age of 7 years and, on average across groups, the onset was at the age of: 3 years 11 months in the 1st group of patients receiving phenobarbital; 3 years 1 month in the 2nd group receiving Topamax; 4 years 6 months in the 3rd group receiving Lamictal. It has been reliably proven that an earlier onset of the disease was detected in children (p <0.01) whose mothers had aggravating factors during pregnancy (threatened miscarriage, intrauterine infections) and pathology during childbirth. The earlier onset of epilepsy was in the group of patients with pronounced focal disorders in the neurological status (p<0.05) and with severe developmental delay in the first year of life (p<0.05).
Patients receiving phenobarbital had the shortest disease duration from the onset of seizures to the start of taking the drug - on average 1 month 24 days, and in 94% of patients phenobarbital was the first drug in the treatment of epilepsy; 6% of patients switched to phenobarbital after unsuccessful treatment with other AEDs.
The disease history looked different in the other two groups. In the group of patients taking topiramate, the average duration of illness before inclusion of the drug in the treatment protocol was 4 years 8 months, and almost all patients (94%) had already received previous therapy with other AEDs. In 61% of cases, combination antiepileptic therapy was used. In the group of patients taking lamotrigine, the average duration of the disease was 5 years 2 months and all patients also had previous antiepileptic therapy before taking the drug. In 90% of cases in the study, lamotrigine was used in combination with other AEDs. It has been statistically proven that the longer the duration of the disease, the more different AEDs were used in the treatment of patients (p <0.05).
In all three groups, the majority of all patients had severe neurological pathology in the form of spastic paresis, pathology of cranial nerve function, cerebellar symptoms, mental retardation - from 71 to 87% of patients. Consequently, a delay in the development of children in the first year of life was observed in 65-76% of patients (see Table 1). Analyzing the observed seizures, it was revealed that secondary generalized seizures predominated in all groups, which accounted for 40-50% of all types. They were more often recorded in children with an earlier onset of the disease (p <0.01).
When assessing the effectiveness of the studied drugs, results were obtained that are summarized in table. 2.
Complete cessation of attacks when using drugs of the old generation (phenobarbital) and the new generation (Lamictal and Topamax) occurred in the same number of patients in each of the study groups - in 26% of cases. However, there were differences in rates of reduction in seizure frequency of 50% or more. The highest result was obtained in patients taking topiramate - 19 (61%). In patients taking lamotrigine, this figure was slightly lower - 14 (45%) patients. And in only 4 (12%) patients treated with phenobarbital, attacks were reduced by 50% or more. Accordingly, this group had the highest percentage of cases in which the effect was absent or minimal - 21 (62%) patients.
From the data presented it is clear that the most pronounced positive effect in the form of complete relief of attacks or their cessation by 50% or more was achieved in the group of patients receiving topiramate - 87% (27 patients). In the group of patients taking lamotrigine, this figure was 71% (22 patients). And the effect was the smallest in patients treated with phenobarbital - 38% (13 patients). We did not find any significant differences in the registration of positive dynamics on the EEG in all three groups. A decrease in the epileptiform activity index was noted in 8 (24%) patients taking phenobarbital, 8 (26%) patients taking Topamax, and 11 (35%) patients taking Lamictal. The presence of differences in the effectiveness of treatment in the three study groups was statistically confirmed.
Discussion
We compared the results of our study with the data available in the literature. In this regard, we recall that during treatment with phenobarbital, we noted persistent clinical remission or complete cessation of attacks in 26% of cases and a reduction in the frequency of attacks by 50% or more in 12% of patients. Thus, a positive effect was achieved in the overall group in 38% of patients. Low effectiveness of treatment was observed in 62% of patients. Our results correlate with the studies of many authors. In his observation, S.R. Boldyreva [2] notes complete control of seizures in 33% of patients. In the review by O.A. Pylaeva et al. [11] compared the effectiveness and tolerability of barbiturates and valproate in children aged 6 months to 15 years. Valproates were used in doses of 30-50 mg/kg per day, barbiturates - in an average therapeutic dose of 2-5 mg/kg per day. The therapeutic effect in monotherapy was registered in 65% of cases when taking valproate and only in 30% of cases when taking barbiturates. In 75% of cases, the improvement in the patients' condition while taking barbiturates was temporary, and subsequently the frequency of attacks increased again. In the work of A.A. Dautova et al. [5] conducted a comparative analysis of monotherapy for epilepsy with phenobarbital and diphenine in patients with different forms of epilepsy. Phenobarbital was used at an average daily dose of 2.0-2.5 mg/kg per day, and diphenin at a dose of 4-5 mg/kg per day. As a result, attacks stopped in 7 (16%) of 44 people receiving phenobarbital, and in 35 (out of 60) receiving diphenine. In a study by W. Wang et al. [58] included 2455 patients. After 24 months from the start of treatment with phenobarbital, 26.2% of patients were seizure-free and 31.3% of patients had a reduction in seizures of 75% or more.
Meanwhile, some authors note higher efficiency in treatment with phenobarbital. Thus, in a study by D. Pal et al. [45] phenobarbital was used in the treatment of children with different types of seizures at an average therapeutic dose of 3 mg/kg per day, and within 6 months complete clinical remission was achieved in 68% of cases. In the observation of C. Valvi et al. [54] good seizure control was achieved in 84.8% of children treated with phenobarbital. In a study by K. Nimaga et al. [44] phenobarbital was used in the treatment of children at doses of 50 mg/day and in adults at a dose of 200 mg/day. After 1 year from the start of treatment, 80.2% of patients were seizure-free for 5 months. And 15.7% of patients had a significant reduction in seizure frequency. In the work of S. Ismael [31], phenobarbital was used in the treatment of children aged 5 months to 12 years. A good treatment result was observed in 62.4% of children, a reduction in seizures by 50% was registered in 7.69% of children. In a study by N. Thilothammal et al. [52] compared the effectiveness and tolerability of phenobarbital, diphenine and valproic acid preparations. It was shown that the effectiveness of treatment was relatively similar in all three groups.
We have not identified a preferential effect of phenobarbital in relation to different forms of epilepsy and different types of epileptic seizures (p>0.05). There were no significant differences in its effectiveness between symptomatic focal forms and cryptogenic (presumably symptomatic) focal forms of epilepsy (p>0.05). It was found that the duration of the disease before starting phenobarbital also does not affect the effectiveness of therapy. In patients with a long duration of the disease, the effectiveness of treatment was comparable to patients with relatively recent illness (p>0.05). But S. Ismael [31] established the greater effectiveness of phenobarbital in generalized convulsive attacks (a positive effect was noted in 72.6% of patients); it was less effective for focal attacks - 43.75% and for focal attacks with secondary generalization - in 47.05% of patients.
In the group of patients receiving topiramate therapy, complete cessation of attacks was observed in 26% of patients and a decrease in the frequency of attacks by 50% or more was recorded in 61% of patients. We compared our results with those of other authors. In the study by K.Yu. Mukhina et al. [8] complete relief of seizures was noted in 19.5 and 16.5% (36% together, respectively) in patients with symptomatic focal forms of epilepsy. According to H. Bootsma et al. [20], this figure is up to 20% of patients. And in the work of Y. Cho et al. [23] clinical remission was noted in 30.4% of cases. Relatively low efficiency was noted in the study by K. Krakow et al. [34] - only 10% of patients over 12 years of age were completely free from seizures. S. Grosso et al. [27] also noted the cessation of seizures in 13% of patients out of 59 treated children under 2 years of age. In the work of S. Grosso et al. [28] studied the effectiveness of topiramate in children with refractory forms of epilepsy. Seizures were controlled in only 4% of children. On the contrary, a high therapeutic effect was noted in the work of domestic authors. In the observation of K.Yu. Mukhina et al. [7] high clinical efficacy was achieved in 46.5% of patients. A.B. Hecht et al. [4] noted drug remission lasting more than 1 year in 47.8% of patients. K.V. Voronkova et al. [3] recorded complete clinical remission in 48% of patients. In the study by S.R. Boldyreva [2] obtained complete control over attacks in 53% of patients.
A positive effect overall in our study was obtained in 87% of patients. In the work of K.V. Voronkova et al. [3] this figure was 92%. In the work of K. Krakow et al. [34] a good result was observed in 82%. In the observations of other authors, the positive effect of treatment was approximately the same - 58.5-73.2%. Increased frequency of attacks was registered in 3% of patients [2] and in 6% of cases [8], which is comparable to the results of our study - 3%.
Topiramate showed better results in the treatment of patients with cryptogenic focal forms of epilepsy. However, in the work of S. Al Ajlouni et al. [15] did not reveal any difference in the effectiveness of treatment for symptomatic and cryptogenic forms of epilepsy. We noted a positive result in the treatment of multifocal forms of epilepsy - 86% of patients had a complete cessation of seizures or a reduction in their frequency by 50% or more. In the group of patients with focal frontal epilepsy, 6 (75%) showed a reduction in seizures by 50% or more. In only 1 (12.5%) case the attacks stopped completely. In the study by K.Yu. Mukhina et al.[8] a positive effect when taking topiramate in patients with symptomatic frontal lobe epilepsy was observed in 63% of cases. A. Verrotti et al. [57], based on observations of a representative group of patients, also note the effectiveness of topiramate in the treatment of frontal lobe epilepsy in monotherapy.
Topiramate showed greater effectiveness in patients with frequent serial tonic axial spasms that occur during night sleep, with focal hypermotor seizures, as well as with secondary generalized convulsive seizures (p>0.05). Identical effectiveness was noted in their observation by K.Yu. Mukhin et al. [8]: the drug was highly effective against focal motor, tonic and secondary generalized seizures. In some cases, Topamax clearly reduced the frequency of atypical absence seizures. However, in monotherapy, its insufficient effectiveness was noted in myoclonic and automotor seizures. It has been proven that the effectiveness of therapy was higher in patients who had a short history of the disease before inclusion in Topamax therapy (p = 0.006).
In our study, 61% of patients were treated with topiramate in combination with other AEDs. In our observation, combination therapy with topiramate and valproic acid drugs turned out to be the most rational and effective. The literature also provides data on the effectiveness of topiramate in combination therapy with various AEDs. In the work of A. Schreiner et al. [47] used a combination of topiramate with valproic acid preparations. At the end of the study, 70% of patients were switched to Topamax monotherapy at a dose of 150 mg/day. In 51% of patients, the attacks stopped completely; in general, a positive effect was noted in 75% of patients. In comparison, the work of A. Kowalik et al. [33] topiramate has been successfully used in combination therapy with carbamazepine and oxcarbazepine. At the end of the study, 73% of patients were switched to alternative monotherapy with topiramate at a mean dose of 100 mg/day. A positive effect was noted in 91% of patients, of which 62% were completely free of attacks.
In the group of our patients receiving lamotrigine, stable clinical remission was achieved in 26% of cases and a reduction in the frequency of attacks by 50% or more was observed in 45% of children. In 6% of cases, an increase in seizures was observed. Overall, a positive effect was achieved in 71% of patients. Higher efficacy was observed in the group of patients who had a later onset of the disease (p=0.037). On the other hand, children who had a high seizure frequency (p=0.031) and had received prior treatment with multiple antiepileptic drugs (p=0.008) had less treatment benefit.
Similar results were obtained in the work of R. Jain et al. [32]: in 20 children (average age 90 months), lamotrigine was used in combination therapy with valproic acid drugs in an average therapeutic dose of 3.86 mg/kg. A significant positive result was observed in 72% of patients, of which 27.7% of patients had a complete cessation of attacks and 44.4% of patients had a significant reduction in the frequency of attacks by 50% or more. In a study by S. Zubcevic et al. [61] included 61 children who began receiving combination therapy with lamotrigine an average of 16 months after the onset of the disease. In 67.2% of cases, the seizures were focal and secondary generalized convulsive. As a result, a decrease in the frequency of attacks by 75-100% was noted in 37.7% of patients, and by 50-75% in 21.3% of patients. In general, summing up these data, we can indicate a positive effect in treatment in 59% of children. In a study by Ch. Song et al. [49] when using lamotrigine in additional therapy in 114 patients, seizure relief by 50% or more was observed in 57% of patients; subsequently, 22.8% of patients were transferred to monotherapy with this drug. In the publication by M.B. Mironova et al. [6] paid special attention to the use of lamotrigine in additional therapy to valproic acid drugs in 38 patients with various forms of epilepsy aged from 3 to 25 years (average age 14.3 years). A positive effect with the addition of lamotrigine was noted in 73.8% of cases: complete therapeutic remission in 36.9% of cases, a reduction in the frequency of attacks by 50% or more in 26.9% of cases. High efficiency while taking lamotrigine (100% relief of attacks in 54% of patients) was registered by H. Arif et al. [16]. Particular attention to the gender characteristics of the use of lamotrigine was paid to G.N. Avakyan et al. [1], emphasizing the positive effect of the drug on reducing the frequency of attacks.
In our work, lamotrigine showed good results in the treatment of frontal lobe epilepsy: in 58% of patients there was a significant decrease in the frequency of attacks and in 17% of cases the attacks were completely stopped. Lamotrigine showed greater effectiveness in patients with focal motor hemiclonic seizures, as well as in secondary generalized convulsive seizures (p>0.05). N.Yu. Perunova et al. [9] noted the effectiveness of therapy in patients with partial seizures in 8% of cases and with secondary generalized ones in 12% of cases. In their observation, E. Trevathan et al. [53] state the effectiveness of lamotrigine in children with partial seizures, absence seizures, and various types of seizures in Lennox-Gastaut syndrome. In a study by S. Zubcevic et al. [61] Lamotrigine was less effective in partial seizures. According to R. Jain et al. [32], lamotrigine showed a good effect in all types of seizures except myoclonic ones.
In our study, in 90% of cases, lamotrigine was used in combination therapy with other AEDs with high efficiency (p = 0.01). In 52% of cases, a combination of lamotrigine with valproic acid drugs (Depakine) was prescribed. A similar observation was noted in the work of N.Yu. Perunova et al. [9]: during combination therapy with lamotrigine and valproate, a reduction in the frequency of attacks by 50% or more was observed in 48.5% of patients.
Thus, new generation AEDs, having a complex mechanism of action on the processes of epileptogenesis, have shown higher effectiveness in the treatment of focal forms of epilepsy in children. If we take into account that the main goal of pharmacotherapy for epilepsy is the complete cessation of seizures without the appearance of neurological, cognitive and somatic side effects and ensuring educational, professional and social adaptation of patients, then the creation of new broad-spectrum AEDs allows expanding therapeutic options in the treatment of epilepsy, especially focal and pharmacoresistant forms.
Features of clinical manifestations of epilepsy depending on the location of the pathological focus
Temporal lobe epilepsy is the most common form of focal epilepsy. The epileptogenic focus is located in the temporal lobe of the brain. Focal temporal lobe epilepsy is characterized by seizures with loss of consciousness, the presence of automatisms and an aura. The attack lasts on average 30-60 seconds. Oral automatisms are characteristic of children, and gesture-type automatisms are characteristic of adults. Paroxysms of focal temporal lobe epilepsy have a secondary generalization. Post-ictal aphasia is recorded when the dominant hemisphere of the temporal lobe of the brain is damaged.
The epileptic focus of frontal focal epilepsy is located in the frontal lobe of the brain. This focus provokes short-term paroxysms, which can occur in series. Frontal FE is not characterized by an aura. Symptoms of frontal FE: turning the eyes and head to one side, complex automatic gestures, emotional arousal, screaming, flinching, aggression. If the focus of the pathology is in the precentral gyrus, then motor paroxysms of Jacksonian epilepsy appear. Epileptic seizures of frontal focal epilepsy occur during sleep.
When the lesion is located in the occipital lobe of the brain, epileptic seizures occur, accompanied by visual impairment. For example, narrowing of visual fields, visual hallucinations, ictal blinking, transient amaurosis, etc. The duration of visual hallucinations is 13 minutes.
The location of the focus of epileptic activity in the parietal lobe is rare. The parietal lobe is affected in the presence of a tumor or cortical dysplasia. Parietal focal epilepsy is characterized by simple somatosensory paroxysms: short-term aphasia or Todd's palsy. If the area of epileptic activity is localized in the postcentral gyrus, then Jacksonian seizures may occur.
Nonconvulsive manifestations
These are the most difficult seizures to diagnose.
Can occur in children and adults. Symptoms can manifest themselves in the form of mental pathology - clouding of reason. This form responds very poorly to drug therapy. Symptoms:
- short-term attacks with “switching off” consciousness;
- change in facial expressions;
- vivid manifestations of pathological joy or negative emotions: rage, even suicide;
- delirium, visual hallucinations, confusion and nonsense of speech.
This form of the disease is the most dangerous, because it is often undiagnosed and untreated.
Diagnosis of focal epilepsy
If partial paroxysm occurs for the first time, the patient needs a detailed examination, since this symptom may be a manifestation of serious cerebral pathology. During the consultation, the neurologist asks to fully describe the duration, nature, and sequence of development of the epileptic attack. The identified deviations help to establish the location of the pathology focus.
Epileptic activity is diagnosed using EEG. Epi-activity of focal epilepsy can be recorded on the EEG even during the interictal period. If the EEG without an attack is uninformative, then electroencephaloscopy should be performed with provocative tests and/or during an attack. Subdural corticography is an electroencephaloscopy with the installation of electrodes under the dura mater. Helps to accurately determine the location of the pathological focus.
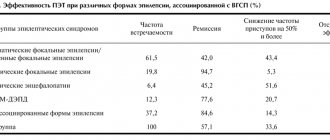
Of the instrumental research methods, the most effective for elucidating the morphological basis of PE is MRI. The thickness of the sections should be about 1-2 mm in order to detect the smallest defects in the brain substance as accurately as possible. In the case of symptomatic epilepsy, MRI makes it possible to identify the root cause: focal lesions, atrophy, dysplasia. In some cases, such changes cannot be detected, and then the diagnosis is idiopathic or cryptogenic PE. As an additional, but optional study, a PET scan of the brain may be prescribed. PET will document the epileptogenic region as an area of increased metabolic activity. SPECT is also used as an additional diagnostic method, during which a specialist is able to monitor the dynamics of perfusion of the cerebral substance: during a seizure, a picture of hyperperfusion of the epileptogenic focus will be observed, and in the period of time between paroxysms, hypoperfusion will be observed.
What are pseudoepileptic seizures?
Pseudoepileptic seizures (conversion, psychogenic, non-epileptic or hysterical seizures) are sometimes difficult to distinguish from epilepsy by external signs; however, in some patients a combination of epileptic and non-epileptic seizures is possible. The incidence of these disorders is quite high, they account for approximately 20% of all cases considered to be resistant forms of epilepsy. Attacks of this type are more common in women. The family history of patients often contains indications of mental disorders. It is more correct to consider these attacks as a manifestation of mental illness; they are not accompanied by epileptic activity on the EEG and are not controlled by anticonvulsants (unlike epileptic seizures). Pseudoepileptic seizures can have different external manifestations. Pseudoepileptic seizures can take a status course, in which case there is a risk of introducing large doses of anticonvulsants into the body and even the use of mechanical ventilation.
As a rule, these attacks do not occur when the patient is alone; witnesses are needed for their occurrence; attacks are usually provoked by a conflict situation. As a rule, during such attacks the patient does not receive injuries (even if he falls), does not bite his tongue, and there is no urinary incontinence. The onset of pseudoepileptic seizures is often gradual, cyanosis (bluish discoloration of the skin) is not observed. Convulsions in the limbs are less rhythmic than during epileptic seizures and may have the character of asynchronous, chaotic movements. The patient actively resists attempts to open his eyes during the attack. Post-ictal drowsiness or confusion is not common.
The main method of differential diagnosis is video-EEG monitoring.
Unlike malingering, patients cannot specifically induce seizures and control them. Pseudoepileptic seizures are also a disease that requires treatment, however, treatment is carried out not with anticonvulsants, but with psychotropic drugs.
Therapy for focal epilepsy
Treatment of focal epilepsy is prescribed by a neurologist and epileptologist, and includes taking anticonvulsants. Anticonvulsants include: carbamazepine, topiramate, phenobarbital, levetiracetam, etc. For the treatment of parietal and occipital epilepsy, pharmacotherapy will be sufficient. In case of focal temporal lobe epilepsy, after 1-2 years of therapy, resistance to anticonvulsant treatment may occur. If there is no effect from therapy, the doctor prescribes surgery.
The operation is performed by neurosurgeons and is aimed at complete removal of the focal formation (malformation, tumor, cyst) or partial removal of the epi-site. Focal resection is used if the focus of epileptic activity is well localized. If cells that are also a source of epileptic activity are adjacent to the epileptogenic zone, then extended resection is prescribed.
Prognosis of focal epilepsy
The prognosis of focal epilepsy depends on its type.
Since idiopathic epilepsy is benign and occurs without cognitive impairment, the cessation of paroxysms occurs spontaneously in adolescence.
Cerebral pathology determines the prognosis of symptomatic epilepsy. Unfortunately, it is unfavorable for severe brain malformations and tumors. This epilepsy is manifested by mental retardation, which is observed with early onset of epilepsy.
In 60-70% of patients after surgical treatment there is a decrease or absence of epiparoxysms. Complete disappearance of epilepsy after a long time is recorded in 30%.