Pathological characteristics
An epidural hematoma is a lens-shaped area of blood in the epidural space (arrowheads in Fig. 3,4)
Fig.1
Fig.2
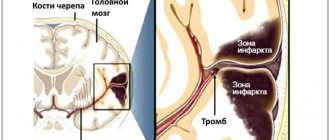
Acute epidural hematoma is a limited lens-shaped zone of high density (60-80 HU) along the convexital surface of the frontotemporal region of the cerebral hemisphere on the right (arrow heads in Fig) with air bubbles (arrows in Fig), which is an indirect sign of a fracture, which is visualized on CT in bone window (arrowheads in Fig) and causes damage to the middle meningeal artery, whose bleeding forms an epidural hematoma.
Fig.3
The dura mater is tightly attached to the skull bone in the area of the sutures, thus, the blood does not extend beyond the boundaries of the skull sutures in the epidural space and forms a biconvex lens shape. The figure visualizes a small-volume epidural hematoma located infratentorially along the convex of the left cerebellar hemisphere (arrowheads).
Fig.4
On MRI, an epidural hematoma is lens-shaped (arrowheads in Fig.) and the intensity of the MR signal depending on the time elapsed from the moment of occurrence (in Fig., early subacute epidural hematoma is hypointense in T2 and T2*, hyperintense in T1 in the periphery, hypointense in T1 in the center ), located under a linear fracture of the left half of the squama of the occipital bone (arrow).
Fig.5
Figure shows a combination of an epidural hematoma of the left occipital region (arrowheads in Figure), a contusion focus of the left frontal lobe (arrows in Figure) and a left-sided subdural hematoma (dotted arrow in Figure).
In 30% of cases, epiural hematoma is combined with subdural hematoma and contusion lesions, and with skull fractures in 90% of cases. In the occipital region on the left (the arrowheads in Fig. are infratentorial, in Fig. supratentorial) the contusion focus is determined directly under the fracture of the squama of the occipital bone, in the place where the blow occurred, and the anti-shock (the direction of the force vector is the dotted arrow in Fig.) contusion focus is determined strictly oppositely at the pole of the right frontal lobe (arrows in Fig).
Fig.6
Clinical picture
The typical clinical picture of an epidural hematoma has a pronounced light gap. The patient briefly loses consciousness, and after recovery there may be a slight daze. During this period, the victim complains of weakness, dizziness, and mild headache. Upon examination, retro-, congrade amnesia, slight anisoreflexia, slight asymmetry of the nasolabial folds, spontaneous nystagmus, and minor meningeal manifestations are determined. The patient's condition may be assessed as mild or moderate. The light period can last from 30 minutes to several hours.
At the end of the light period, a rapid deterioration of the patient’s condition develops. The headache becomes intense, nausea and vomiting appear. Upon examination, a change from psychomotor agitation to worsening disorganization of consciousness is visible - from stupor to stupor, coma. A rapid decline of consciousness with a sharp transformation into coma is possible. The patient’s heart rate slows down, blood pressure decreases, and on the nervous system side, there is progressive brachiocephalic paresis (paresis of the facial nerve, decreased muscle tone in the upper extremities) on the opposite side of the hematoma. In the direction of the hematoma - dilation of the pupil, subsequently lack of reaction to light.
During the progression of epidural hematoma, focal symptoms (paresis, anisocoria) are often the first to appear, ahead of the formation of brain compression.
Often there is an erased light period with the development of an epidural hematoma. In this case, a coma immediately sets in, and the TBI is classified as “severe.” After a few hours, the coma may be replaced by stupor.
In this state, you can enter into verbal contact with the patient, during which he complains of an intense headache. On examination, mild or moderate hemiparesis is recorded. A minor light episode can last from a few minutes to a day.
After this period, the patient’s condition worsens, excitement increases and turns into a coma, symptoms of paresis intensify, and complete plegia of the limbs opposite the hematoma may develop. Tonic muscle spasms of unilateral limbs, disturbances of vestibular and oculomotor functions, and signals of brain stem damage may be observed. In terms of vital functions, significant disorders may be detected.
An epidural hematoma can develop without a clear period, although such options are rare. This condition can develop in severe TBI with numerous brain lesions. The onset of a coma is noted immediately after the injury and does not change.
Subacute development of epidural hematoma is determined by a long light episode - up to 10-12 days. The patient remains conscious. On examination, there is a tendency to bradycardia and the occurrence of some focal manifestations.
There may be a wave-like aggravation of the patient’s condition, disorganization of consciousness to the point of deep stupor. Before this, nervous excitement and intense headache appear. With ophthalmoscopy, congestive optic discs are recorded, which indicate compression of the medulla.
Focal symptoms of epidural hematoma depend on its location. Hemorrhages in the parasagittal zone are manifested by pyramidal disorders with the greatest predominance of paresis in the foot. An epidural hematoma localized in the frontal lobe causes mental disturbances and minor focal symptoms. The occipital lobe affected by epidural hemorrhage results in loss of visual fields on the affected side—homonymous hemianopia.
Characteristics of the phases of development of epidural hematoma
Acute phase (from the end of 1 day to 3 days)
Acute epidural hematoma is characterized by high density on CT (60-80 HU). The MR signal changes (oxyhemoglobin turns into dioxyhemoglobin - magnetic), becomes hypointense in T2 (arrowheads in Fig.) and T2* (arrowheads in Fig.) and hypointense in T1 (arrowheads in Fig.). The best way to diagnose an acute epidural hematoma is a computed tomography scan. Massive epidural hematoma (arrowheads in Fig.), which occurred due to rupture of the transverse sinus of the dura mater (arrow in Fig.).
Fig.7
Fig.8
Fig.9
Subacute phase (from 3 days to 14 days)
Subacute epidural hematoma on CT has a density isodense to the brain and is poorly differentiated. On MRI, the subacute phase of the development of subdural hematoma (transition of dioxyhemoglobin into methemoglobin) is divided into 2 parts: early (3-7 days) and late (7-14 days) subacute phases. The early subacute phase (Figure) is characterized by a hypointense signal on T2 and a hyperintense signal on T1 in the periphery (arrows in Figure). In the center, hypointense T1 remains - oxidation of hemoglobin from the periphery to the center (arrowheads in Figure). In the late subacute stage (Figure), the hematoma is relatively homogeneous and hyperintense in T1 and T2 (due to hemolysis of the erythrocyte containing methemoglobin and its release to form extracellular methemoglobin). At the subacute stage, the hematoma is rich in methemoglobin, which has a hyperintense MR signal on T1 IP, and, due to this property, can produce an artifact on 3D time-of-fly (TOF) sequences, which should be taken into account to avoid diagnostic errors (arrowheads ).
Fig.10
Fig.11
Chronic phase (more than 2 weeks)
Chronic subdural hematoma has a low (cerebrospinal fluid) density of 10-13 HU due to hemolysis of red blood cells and protein breakdown. The MR signal is hyperintense in T1 and T2 due to extracellular methemoglobin, and along the edge of the dura mater there is a border of low MR signal, which is better visualized on a gradient echo T2* - hemosiderin deposition (arrows in Fig). Subepidural hematoma (combination of unilateral epidural and subdural hemorrhage)
Fig.12
Combination of unilateral epidural and subdural hemorrhage
Fig.13
Fig.14
Bilateral epidural hematoma
Fig.15
Consequences of traumatic brain injury and complications
Considering TBI, its consequences and complications, it should be noted that their severity varies from headache to severe neurological symptoms. At different periods of time after a head injury, the following are possible:
- Disorders of consciousness up to coma .
- Meningeal syndrome.
- Post-traumatic arachnoiditis.
- Post-traumatic hydrocephalus .
- Post-traumatic epilepsy .
- Cerebral ischemia.
- Pathological reflexes and paresis .
- Asthenovegetative syndrome.
- Post-traumatic amnesia .
- Post-concussion syndrome , which was mentioned above.
- Akinetic mutism : the patient is awake, but there are no attempts to speak or move.
- Chronic disorders of consciousness - a state of minimal consciousness and a vegetative state.
- Primary post-traumatic dementia manifests itself after recovery from a coma due to severe traumatic brain injury with contusion of the frontal and temporal lobe.
- Persistent neurosis-like syndrome.
- Disorientation and disintegration of speech, behavioral disorders.
- Most patients do not become disabled immediately after receiving an injury, but the consequences of a head injury develop over time - after several months or even years. The long-term period lasts up to 2 years and 70% of victims develop traumatic brain disease and dementia. Post-traumatic dementia can progress over time and resembles Alzheimer's disease . Such complications occur especially often with persistent subdural hematomas.
The operations performed are also accompanied by complications and consequences. The following consequences of removal of a cerebral hematoma can be mentioned:
- Postoperative recurrence of hematomas (especially in chronic subdural hematomas).
- Pneumocephalus (accumulation of air in the skull).
- Secondary hemorrhages into brain tissue.
- The appearance of seizures .
- After surgery, bacterial meningitis (especially after drainage of the ventricles of the brain).
- Pulmonary embolism.
- Thrombophlebitis.
- Septic condition.
- Severe cerebral edema .
Surgical treatment
Fig.22
The book “Brain Tumors. CT and MRI diagnostics” read, view / order
Author: radiologist, Ph.D. Vlasov Evgeniy Alexandrovich
Full or partial reprint of this article is permitted by installing an active hyperlink to the source
- you have lost the description of your MRI or CT scan,
- The doctor was not satisfied with the description of your MRI or CT scan
- you doubt the conclusions based on your MRI results,
You can order a review of your MRI or CT scan with a detailed transcript here:
List of sources
- Zhulev N.M., Yakovlev N.A. Mild traumatic brain injury and its consequences. – M., 2004; 127.
- Zotov Yu. V. Complex treatment of severe TBI, taking into account the nature of brain damage and the severity of hypertensive-dislocation syndrome // Bulletin of Surgery, 1996, No. 1, p. 53-56.
- Clinical Guide to Traumatic Brain Injury / Ed. A. N. Konovalov, L. B. Likhterman, A. A. Potapov. T. 1. M.: Antidor, 1998.
- Dzhindzhikhadze R. S., Dreval O. N., Lazarev V. A. Emergency neurosurgical interventions in patients with intracranial tumors // Problems of neurosurgery. – 2011. – Vol. 3. – pp. 62–71.
- Fraerman A.P., Kravets L.Ya., Sheludyakov A.Yu., Trofimov A.O., Balyabin A.V. Compression of the brain in isolated and combined traumatic brain injury. N. Novgorod; 2008; 324 pp.
Therapy
Conservative treatment
If the volume of the resulting cavity does not exceed 30–50 ml, the patient does not experience progressive symptoms and signs of brain compression, conservative treatment is possible.
The main goal of therapy is the gradual resorption of the mass of spilled blood, and therefore it is vitally important to constantly monitor the dynamics of the hematoma volume.
Surgical intervention
For larger formations and the presence of brain compression, emergency surgical intervention is indicated. In such cases, a milling hole is made in the area of the skull with the expected localization of the accumulation.
The choice of treatment tactics is determined by the degree of damage and its location
Through it, using a special aspirator of blood clots and fluid, part of the hematoma is removed, after which craniotomy is performed to remove the formation in full and ligate the damaged vessel.
If veins are the source of bleeding, they are coagulated, followed by packing with a hemostatic sponge. In cases of damage to the diploic veins, surgical wax is used, and if sinus injuries are detected, their plastic surgery and tamponade are performed. At the end of the operation, a bone flap is put in place and the wound on the surface of the scalp is sutured.
Simultaneously with the manipulations, hemostatic, decongestant and symptomatic treatment is used. During the recovery period, patients receive neurometabolic and absorbable medications. To speed up the process of muscle recovery in paretic limbs, therapeutic massage and physical exercise are indicated.
Closed external drainage
As an alternative to craniotomy, a minimally invasive surgical intervention - closed external drainage - can be chosen. This method is recognized as more gentle and has a number of advantages, but can only be used in cases where emergency removal of the formation is not required.
According to indications, minimally invasive surgery is performed
An intraosseous needle is inserted through the skin to perforate the skull. A special drainage catheter, the diameter of which does not exceed 3 mm, is placed into the resulting hole. A fluid receiver is attached to it, which is placed 15–20 cm below head level to ensure optimal fluid outflow.
When carrying out minimally invasive therapy, the integrity of the cranium is not compromised, and the risk of infection and the likelihood of relapse of the pathology are minimal.