Small focal leukoencephalopathy of vascular origin is a diagnosis that is more often given to male patients who have crossed the age threshold of 55 years, but not everyone knows what it is.
With leukoencephalopathy of the brain, the white matter in its subcortical structures is destroyed.
This type of disease is one of the types of encephalopathy, first described by the German psychiatrist and neurologist Otto Binswanger in 1894, and therefore was named after him.
The unclear description of the disease and the small number of examinations of patients with signs of dementia for a long time prevented many neurologists and psychiatrists from recognizing the pathology.
With the advent of computed tomography and magnetic resonance imaging, changes in the white matter of the brain associated with persistently elevated blood pressure, causing dementia, have been confirmed.
Treatment of the disease is carried out by neurologists and psychiatrists.
Leukoencephalopathy has varieties, but almost always we are talking about changes in the white matter of the brain. The type of disease determines the treatment regimen.
Lecoencephalopathy of vascular origin
Lecoencephalopathy of vascular origin (small focal) is a cerebrovascular pathological process.
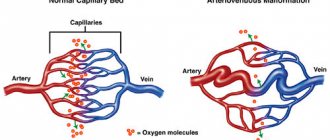
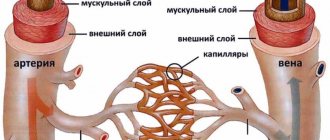
The fundamental factor for the appearance of vascular leukoencephalopathy is constantly elevated blood pressure, as well as its systematic jumps.
Arterial hypertension causes sclerosis of small cerebral vessels - capillaries, their ischemia, thickening of vascular walls, etc. As a result, the white matter that transmits nerve impulses undergoes atrophic changes. In this case it is observed:
- its reduction;
- decrease in density;
- fluid replacement;
- the appearance of numerous hemorrhages, cysts, small foci of destruction.
The ventricles of the brain begin to collapse.
Typically, the first signs of small focal leukoencephalopathy can appear in patients who have reached the age of sixty, and in case of a hereditary factor, even earlier.
Multifocal progressive vascular leukoencephalopathy
Multifocal progressive vascular leukoencephalopathy manifests itself with a viral infection of the central nervous system, which contributes to impaired immune function.
Weakening of the immune defense leads to the destruction of the white matter of the brain.
Pathological changes are provoked by immunodeficiency: HIV-infected (5%), AIDS patients (50%).
Also, modified leukocytes in leukemia lose the ability to fight infection, which contributes to the occurrence of immunodeficiency and, as a consequence, to pathological changes in the brain.
Exposure to viruses leads to focal damage to the sheaths of nerve fibers, enlargement and deformation of nerve cells. There is no involvement of gray matter in the process. The structure of the white matter changes, it softens and becomes gelatinous with the appearance of multiple small depressions on its surface.
This form of the disease poses a danger not only to the health, but also to the life of the patient. It should be noted that modern antiviral therapy has significantly reduced the prevalence of the pathology.
The manifestation of cognitive impairment can range from mild impairment to full-blown dementia. Focal neurological symptoms include: speech impairment and decreased vision, sometimes to the point of complete loss. Accelerated progression of movement disorders can cause severe disability.
Periventricular (focal) leukoencephalopathy
Periventricular (focal) leukoencephalopathy is extensive damage to the brain caused by a lack of oxygen or chronic disruption of its blood supply.
The areas of the cerebellum, brain stem and subdivisions responsible for human movements are affected by pathology. Prolonged lack of oxygen leads to dry necrosis (death) of the white matter. It develops rapidly and contributes to the occurrence of severe motor activity disorders.
Periventricular leukoencephalopathy can be triggered by fetal hypoxia and lead to cerebral palsy (CP).
Leukoencephalopathy - clinical forms, diagnosis
The medical term “leukoencephalopathy” is used to define a group of diseases accompanied by damage to the white matter and a number of deep structures of the brain.
Rapid progression leads to the formation of senile dementia. In children, there are vascular varieties, congenital forms with a long chronic course. The survival time for this type is longer compared to its multifocal counterpart.
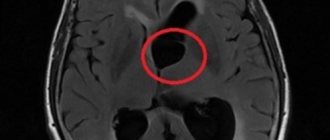
MR image of vascular leukoencephalopathy
To distinguish the pathology from a number of other neurodegenerative diseases with similar clinical symptoms, a classification according to ICD 10 has been developed, which clearly distinguishes the forms of the nosology.
Treatment
Modern medicine does not have methods that can completely rid a person of vascular leukoencephalopathy.
Self-treatment in this case is prohibited. When the first symptoms of the disease appear, it is necessary to urgently contact specialists: neurologists and psychiatrists, who, with the help of therapists, cardiologists, endocrinologists and other specialists, will draw up an individual treatment regimen, taking into account the peculiarities of the course of the pathology and the general health of the patient.
The goal of treatment is:
- slowing down the progress of pathological changes;
- relief of symptoms;
- restoration of the patient's mental state.
Treatment areas include:
- therapeutic measures aimed at combating the development of pathology;
- symptomatic treatment;
- correction of blood pressure, which ideally should not exceed 120/80 mmHg. Art. It should be remembered that the occurrence of hypotension is also undesirable, since a decrease in blood pressure can only aggravate the situation;
- elimination of spontaneous bowel and bladder emptying;
- rehabilitation;
- social adaptation.
Experts use the following medications:
- "Lisinopril" has cardioprotective, vasodilating, hypotensive effects;
- drugs that improve blood circulation in the brain: Cavinton, Pentoxifylline, Clopidogrel;
- nootropics to stimulate mental activity, improve memory and increase learning abilities: Cerebrolysin, Piracetam, Nootropil;
- angioprotectors that restore the walls of blood vessels: “Plavix”, “Cinnarizin”, Curantil”;
- antidepressants: Prozac;
- adaprogens that increase the overall tone of the body: “Aloe extract”;
- vitamins A, E, B;
- in some cases, antiviral drugs: Kipferon, Acyclovir;
- drugs from the group of acetylcholinesterase inhibitors that improve cognitive function: Rivastigmine, Donepezil, Galantamine, Memantine.
Reflexology and physiotherapy are not excluded: breathing exercises, massage, acupuncture, manual therapy sessions.
What is cerebral leukoencephalopathy
Damage to the white matter of the brain is caused in most cases by viruses. Vascular and discirculatory forms are caused by impaired blood supply to a certain area of the brain. Chronic ischemia causes irreversible changes.
Clinical symptoms of the disease more often occur when affected by papillomaviruses. The probability of nosology in patients with HIV is less than 6% according to brain MRI statistics in St. Petersburg.
Forms of vascular origin progress slowly. The chronic course of the disease is characterized by gradual irreversible tissue damage. Mild ischemia provokes the formation of small necrotic areas. Diffuse location leads to neurological disorders.
Clinical picture
Typically, the symptoms of leukoencephalopathy develop gradually. At the beginning of the disease, the patient may be distracted, awkward, and indifferent to what is happening. The patient becomes tearful, has difficulty pronouncing difficult words, and his mental performance decreases.
Over time, sleep problems may occur, muscle tone increases, the patient becomes irritable, involuntary eye movements and tinnitus may occur.
If treatment for leukoencephalopathy is not started at this stage, it progresses: psychoneuroses, severe dementia and convulsions appear.
The main symptoms of the disease are the following deviations from the norm:
- Movement disorders are manifested by impaired coordination of movements, weakness in the arms and legs;
- unilateral paralysis of the arms or legs may occur;
- Speech and vision disorders (scotoma, hemianopsia);
- numbness in various parts of the body;
- swallowing disorder;
- urinary incontinence;
- Epileptic seizures;
- intellectual impairment and mild dementia;
- nausea;
- headache.
All symptoms of damage to the nervous system progress very quickly. The patient may have false wrist paralysis and parkinsonian syndrome, which is characterized by abnormal gait, writing, and body tremors.
Almost every patient experiences memory and intelligence impairment, instability when changing body position or walking.
People usually do not realize that they are sick, so they are often brought to the doctor by relatives.
Types of leukoencephalopathy
The least dangerous form is focal. Formed by chronic inflammatory processes of vascular origin. Lack of microcirculation in a certain part of the brain provokes hypoxia, a lack of oxygen. The death of white matter zones takes several years to develop.
Morphological changes occur more aggressively in hypertension. An increase in intracranial pressure causes ruptures of small capillaries with areas of necrosis of the brain parenchyma. A type of medical language is called “discircular encephalopathy.” Appears in people over 55 years of age.
Progressive multifocal leukoencephalopathy has an aggressive course. People with pathology live no more than 5 years. Lethal outcomes are associated with extensive heart attacks and strokes.
Classification of types of leukoencephalopathy according to ICD 10
A progressive type of vascular origin (Binswanger's disease) is coded with the symbols “I67.3”. Subcortical dementia with code “F01.2” has been excluded from the classification of diseases of the tenth revision.
Progressive multifocal (multifocal) leukoencephalopathy - “A81.2”. The group of the same name includes phenylketonuria, Alexander disease, and Canavan disease. Pathologies of category “IA” are distinguished for reasons, as they are of autoimmune origin - caused by tissue damage by immunoglobulins of the body’s own. Antibodies become aggressive when the structure of the membrane or the genetic information of the cell changes under the influence of viruses, chemical, and physical factors.
Let's look at the complete classification algorithm:
- Diseases of the circulatory system – “IX. 100-199";
- Cerebrovascular diseases “I60-69”;
- Other cerebrovascular diseases - “I67”;
- Progressive vascular leukoencephalopathy – “I67.3”;
- Other specified vascular lesions – “I67.8”.
The international classification of the tenth revision is valid. When encoding the diagnosis, discirculatory encephalopathy, acute cerebrovascular insufficiency, NOS, and cerebral ischemia (chronic) are often encountered.
Clinical symptoms of small focal leukoencephalopathy
Focal symptoms have a subacute course. The initial stages of the disease are identified by neurologists:
- Visual impairment, speech impairment;
- Pathology of the innervation of the muscles of one half of the body;
- Epilepsy attacks;
- Headaches, dizziness;
- Ataxia, anopsia.
Differential diagnosis of focal types is carried out to distinguish them from changes in white matter in HIV and dementia. Spinal lesions occur without impairment of mental functions. Damage to white matter is accompanied by cognitive impairment.
Progressive multifocal leukoencephalopathy
The cause of multifocal white matter damage is the JC virus, which leads to widespread damage to the nervous system. The disease develops against a background of reduced activity of the immune system. Antiretroviral treatment is expensive, so most people die.
Progressive encephalopathy quickly leads to the destruction of the myelin of most nerve cells. The changes are irreversible, the symptoms gradually increase.
About 80% of the country's population are carriers of human polyomavirus type 2, but encephalopathy does not occur. Only immunodeficiencies in AIDS create the possibility of rapid reproduction of the pathogen.
The immunity of older people cannot cope with the activity of polyomavirus (JC) after immunomodulatory or immunosuppressive therapy after treatment of cancer or organ transplant surgery.
In children, the appearance of pathology is observed after the start of therapy for chronic lymphocytic leukemia and Hodgkin's disease.
The 1C virus is transmitted by airborne droplets or fecal-oral routes. The majority of the population is asymptomatic. Provoking factors:
- HIV infection;
- Taking immunosuppressants;
- Lymphogranulomatosis;
- Leukemia.
Magnetic resonance imaging is the only way to identify pathological foci within the white matter. After the appearance of visual impairment, dysarthria, hemiparesis, and aphasia, neurologists will be able to suggest a diagnosis. Final verification is possible only after a microscopic examination of brain biopsies - tissue sections taken from the site of injury.
Until recently, progressive multifocal leukoencephalopathy (PML) was considered a rare, rapidly progressive demyelinating disease of the central nervous system caused by activation of a ring virus of the genus Polyomavirus
family
Polyomaviridae
(polyomaviruses) - John Cunningham virus (John Cunninghamvirus - JC virus), named after the patient in whom it was first discovered in 1971 and the carriers of which are about 80% of the world's population [1–2]. Modern data indicate that PML develops in persons with reduced immunity (immunocompromised persons): with neoplastic diseases (leukemia, Hodgkin's disease, lymphosarcoma, myeloproliferative diseases), tuberculosis, sarcoidosis, with immunodeficiency, AIDS, drug immunosuppression with cytostatics during organ transplantation or neoplasms various localizations [3]. PML often occurs during the treatment of demyelinating diseases with monoclonal antibodies, and the development of PML as a dangerous complication of a systemic inflammatory rheumatic disease against the background of immunosuppressive therapy is also possible [4]. At the same time, in some cases, the development of PML occurs in the absence of severe immunodeficiency. PML is essentially an opportunistic viral infection. For neurologists, the relevance of studying PML is associated with the active use of immunosuppressive therapy and the widespread prevalence of HIV infection. After the introduction of highly active antiretroviral therapy (HAART), the incidence of PML increased and amounted to 1.3 per 1000 HIV-infected persons per year [5].
In clinical practice, early diagnosis of PML is difficult due to the lack of severe symptoms, since infection with the JC virus occurs in childhood and the virus remains in the body for life [6]. The exact location of its persistence has not been fully elucidated—presumably, it is the kidneys and bone marrow. When the immune system is weakened, the virus is transported by leukocytes to the central nervous system, where it begins its replication in the white matter of the brain, or more precisely, in oligodendrocytes. Destruction of the myelin sheaths is macroscopically manifested as multifocal demyelination. The white matter of the cerebral hemispheres is most often affected, but the cerebellum and gray matter may be affected.
Clinical manifestations of PML
Clinical manifestations of PML do not have a specific pattern. The onset of neurological and psychopathological symptoms is subacute (several days) or gradual (several weeks). The first to develop is a rapidly progressing psychotic syndrome. Later, mono- or hemiparesis, speech impairment and loss of visual fields (hemianopsia) appear. Headache, dizziness, ataxia and epileptic seizures are much less common. Characterized by the absence of general infectious and meningeal symptoms. In the early stages of the disease, progressive cognitive impairment occurs, but unlike dementia in HIV infection, it is accompanied by focal neurological symptoms [7]. Patients sometimes experience an atypical variant of the disease (spinal), which occurs without mental impairment [8]. The course is variable, death occurs within 6-12 months [9]. In the terminal stage of the disease, severe dementia, coma and death of the patient develop. The greatest diagnostic difficulties arise in AIDS, when the clinical picture and MRI signs are similar to PML and HIV-associated encephalopathy [10]. In these cases, only detection of JC virus in cerebrospinal fluid (CSF) and brain biopsy allows diagnosis to be made.
Diagnosis of PML
The diagnosis of PML is based on the criteria recommended by the American Academy of Neurology in 2013 [11] (Table 1).
Table 1. Clinical, laboratory and MRI criteria for diagnosing PML
Sometimes, to accurately confirm PML, a biopsy of brain tissue is performed to reveal the classic histopathological triad: an increase in oligodendrocyte nuclei, changes in the size and shape of astrocytes, which become large, bizarre in shape with hyperchromatic nuclei.
Thus, the doctor’s algorithm for suspected PML consists of several stages (Fig. 1)
Rice. 1. Algorithm for a doctor’s action in case of suspected PML. JCV - JC virus. [12].
Differential diagnosis of PML
The differential diagnosis of PML must first be made with infectious encephalopathies ( H erpes simplex
, CMV virus,
Varicella zoster
,
C r y ptocoсcus
,
A spergillus
), with lymphoma, with subacute sclerosing panencephalitis. Unlike the above infections, with PML there are no general infectious and meningeal symptoms.
Drug-induced PML
Taking into account the increasing number of risks under which optimal conditions are created for the proliferation of the JC virus under the influence of drug immunocorrection, the term “drug-induced PML” was proposed [13]. In patients with multiple sclerosis undergoing natalizumab therapy, it is necessary to exclude exacerbation of the underlying disease (with MRI control, the lesions will intensively accumulate the contrast agent) [14, 15]. Attention should also be paid to patients receiving rituximab. The risk of developing PML in patients receiving rituximab is 1:8000 [16]. Rituximab is a monoclonal antibody drug against CD20 precursors of B lymphocytes and mature B lymphocytes. Approved for use in cellular non-Hodgkin's lymphoma and resistant rheumatoid arthritis. Approved by the FDA in 2006 for the treatment of systemic lupus erythematosus. Currently, to monitor the effect of rituximab and identify the side effects of the drug, a special project has been created (Research on Advers Drag Events and Report - RADAR) with the participation of virologists, oncologists, neurologists and other specialists [17]. In early 2014, the Federal Service for Surveillance in Healthcare of the Russian Federation published a letter reporting 2 cases of PML in patients with systemic lupus erythematosus who received belimumab in the post-registration period.
In 2015, L. Calabrese et al. [18] proposed a conditional risk gradation developed for drug-induced PML. Class 1 included natalizumab and efalizumab (discontinued) as drugs with a high risk of developing PML (1/10,000-1/100). Class 2 (low risk) includes rituximab, belimumab, azathioprine, mycophenolate mofetil, methotrexate. Class 3 (very low risk) included tumor necrosis factor-α (TNF-α) inhibitors, abatacept, tocilizumab, anakinra, ustekinumab, and tofacitinib. The use of these drugs requires special careful monitoring of patients and the need to inform them about the risk of developing PML.
PML therapy
Specific therapy for PML has not yet been developed. The following drugs are used for treatment: antivirals, cytostatics, serotonin receptor antagonists, HAART therapy in HIV-infected patients [19]. With the development of this pathology, it is advisable to reduce the dose of glucocorticoids and cytostatic drugs as much as possible. A positive effect has been described from a combination of plasmapheresis (5 sessions every other day) followed by the aminoquinoline drug meflocin and mirtazapine (an antidepressant, a serotonin reuptake inhibitor, which slows the spread of the JC virus by blocking specific receptors) [19]. Symptomatic therapy (decongestant, neuroprotective, antioxidant) is very often carried out.
Thus, PML is a disease that can be encountered in the practice of a neurologist. It can simulate acute cerebrovascular accident (ACVA), chronic cerebral ischemia with severe cognitive impairment. The clinician should constantly remember that the development of this pathology is possible in patients with demyelinating diseases, HIV, and neuroinfections.
We present data from our own observations.
Observation 1.
Patient A.
, 35 years. Entered the City Clinical Hospital No. 15 named after. O.M. Filatov in the direction of emergency medical services (EMS) with a diagnosis of stroke. Upon admission, he complained of spatial disorientation, emotional lability, and decreased memory for current events.
Life history: married, has two healthy children. He has been smoking since he was 17 years old. Two years ago, after a business trip to Thailand, I lost weight sharply, which I attributed to the transition to a “healthy lifestyle”: a balanced diet, intense training. I began to get colds more often. I didn’t go to the doctors.
History of the disease: according to the patient and his wife, about 6 months ago he suffered from right-sided bronchopneumonia (diagnosed during a CT scan of the lungs).
The deterioration in health, according to the patient’s wife, began on January 12, 2017, when dizziness and decreased memory for current events occurred. According to the patient's wife, blood pressure (BP) ranged from 90/60 to 140/90 mm Hg. Hospitalized in the intensive care unit (ICU) for patients with stroke on January 19, 2017. After stabilization of his condition, on January 20, 2017 he was transferred for further treatment and examination to the neurological department.
The condition upon admission is of moderate severity, the skin and visible mucous membranes are of normal color and moisture. There is hard breathing in the lungs, no wheezing. Respiratory rate (RR) 16 per minute. Heart sounds are muffled, rhythmic, heart rate (HR) 76 beats/min, blood pressure 115/80 mm Hg. The abdomen is soft, painless on palpation in all parts. Self-urination. Urine is light and transparent.
Neurological status: conscious, contactable, oriented. Has difficulty finding words when answering simple questions. There are no meningeal signs. Palpebral fissures S
=
D
.
Pupils S
=
D.
_
Photoreactions and corneal reflexes are preserved. Full movement of the eyeballs. There is no nystagmus. The facial muscles are symmetrical. Swallowing is not impaired. Tongue in the midline. There are no paresis. Muscle tone D
=
S
, not changed.
Tendon reflexes D
=
S
, low. Babinski reflex on both sides. Coordinator tests are performed satisfactorily. There are no sensory disorders, the functions of the pelvic organs are controlled.
Complete blood count: lymphopenia up to 1.9-3.2·109.
Antibodies to hepatitis C (aHCV) were detected. Immunoblot (IB) + dated 01/26/17 No. 136240.
The electrocardiogram (ECG) shows normal sinus rhythm, heart rate 57 beats/min. Incomplete blockade of the right bundle branch. Ultrasound examination (ultrasound) of the abdominal organs and kidneys showed signs of splenomegaly and diffuse nonspecific changes in the liver. Electroencephalogram (EEG) without any features.
Multislice CT (MSCT) of the brain dated January 19, 2017: the picture may correspond to an ischemic zone in the right parieto-occipital region. MSCT of the brain dated January 20, 2017: The CT picture (compared to the MRI dated January 18, 2017, presented by the patient) more likely corresponds to leukoencephalopathy after a neuroinfection. Less likely is PML. MRI of the brain dated January 21, 2017: a picture of progressive multifocal leukoencephalopathy of the brain. Signs of intracranial hypertension. In Fig. 2
Rice. 2. MRI of the brain of patient A., 35 years old. a, b — Flair mode; c, d — T2 mode.
Rice. 2. MRI of the brain of patient A., 35 years old. (end) d, f - DVI mode. Tomograms are sequentially arranged in Flair, T2-weighted image and DVI modes.
Based on the clinical picture, positive IB and characteristic changes on MRI of the brain, the patient underwent a PCR test of the CSF for the JC virus to clarify the diagnosis, which turned out to be positive.
The patient was diagnosed with focal brain lesion (PML). IB (+) dated January 26, 2017 No. 136240.
Symptomatic treatment was carried out: Mexidol (ethylmethylhydroxypyridine succinate) 500 mg intravenously (iv); diacarb (acetazolamide) 500 mg/day; Cortexin (polypeptides of the cerebral cortex of cattle) 10 mg intramuscularly (i.m.); recognan (citicoline) 1000 mg i.v. Against the background of the therapy, the condition is showing positive dynamics. A.D.’s indicators have stabilized. There are no complaints of memory impairment. The patient is recommended to continue treatment in a specialized treatment and prevention facility. The described clinical case deserves special attention due to the fact that the socially prosperous patient did not know or deliberately concealed the cause of his health problems; upon entering the clinic, he received treatment for a vascular disease and the consequences of a neuroinfection. Unfortunately, enzyme-linked immunosorbent assay (ELISA) is a laboratory test that allows one to determine the presence of HIV antibodies in the blood and often gives false-positive results. To clarify the diagnosis, an immunoblot was performed for antibodies to HIV and HCV. According to WHO recommendations, immunoblotting (Western blot) is used in the diagnosis of HIV infection as an additional expert method, which should confirm the results of ELISA. Immunoblotting is an expensive and time-consuming technique. The results of this analysis were obtained almost before the patient was discharged from the hospital. At the same time, the prescription of symptomatic therapy made it possible to stabilize the patient’s condition and improve cognitive functions. The choice of drugs was based on the principles of treatment of vascular lesions of the brain.
Observation 2.
Patient R
., 52 years old. Entered the City Clinical Hospital No. 15 named after. O.M. Filatova with a diagnosis of stroke.
When I received the complaint, I could not formulate it due to speech impairments.
Life history: married. He has been abusing alcoholic beverages for a long time and has been smoking since he was 16 years old. Arterial hypertension for the last 10 years.
History of illness: considered himself practically healthy. In August 2016, he noticed dizziness and double vision. An outpatient MRI of the brain with contrast was performed, and he was consulted at City Clinical Hospital No. 24, where he was diagnosed with multiple sclerosis in question, mass formation of the brain, ischemic stroke. He was treated in a hospital with a diagnosis of transient ischemic attack. He completed a course of vascular and neurometabolic therapy with an unstable positive effect. He did not take recommended therapy (antiplatelet agents, statins, antihypertensive drugs). The deterioration of the condition was noted on November 16, 2016; at about 14:00 speech was impaired. Due to the lack of improvement in his condition, he was hospitalized by the ambulance team at City Clinical Hospital No. 15 named after. O.M. Filatova.
The condition upon admission is of moderate severity, the skin and visible mucous membranes are of normal color and moisture. There is hard breathing in the lungs, no wheezing. NPV 16 per minute. Heart sounds are muffled, rhythmic, heart rate 68 beats/min, blood pressure 160/90 mm Hg. The abdomen is soft, painless on palpation in all parts. Self-urination. Urine is light and transparent.
Neurological status: conscious, limited access to productive contact due to severe speech disorders. Cognitive functions cannot be assessed. There are no meningeal signs. Palpebral fissures S
=
D
.
Pupils S
=
D.
_
Photoreactions and corneal reflexes are preserved. Full movement of the eyeballs. Nystagmus and diplopia are absent. The facial muscles are symmetrical. Swallowing is not impaired. Tongue in the midline. In the test, Barre penetrates his right hand. There are no paresis. Muscle tone D
=
S
, not changed.
Tendon reflexes D
>
S
, low. There are no pathological foot signs. Sensitivity could not be reliably assessed due to speech impairments; the functions of the pelvic organs are monitored. No clear disturbances of sensitivity and coordination were identified. The Romberg test was not performed. Stroke scale score (NIHSS) - 2 points.
Laboratory research methods: without features, aHCV, hepatitis B virus surface antigen (HBsAg), antibodies to Treponema pallidum
not detected. ELISA method: aHCV - “+”, IB - “+” from 11/23/16.
The ECG shows a regular sinus rhythm, heart rate 78 beats/min. According to EEG data, against the background of moderate diffuse changes, there is dysfunction of nonspecific brain structures. No paroxysmal activity or focal pathology was detected. Ultrasound of the abdominal cavity and kidneys revealed signs of diffuse nonspecific changes in the liver, pancreas and left kidney cyst.
According to the speech therapist, the patient has amnestic-semantic aphasia, afferent motor aphasia, mixed agnosia, dyscalculia, a component of dysarthria, and a defect of moderate severity.
Neuroimaging: MSCT did not reveal any fresh areas of ischemia or hemorrhage at the time of the study. MRI of the brain shows a picture of subacute ischemia in the left parietal lobe of the brain, signs of central pontine myelinolysis. Encephalopathy. Signs of intracranial hypertension. If clinical and laboratory data are available, PML may be consistent. In Fig. 3
Rice. 3. MRI of the brain of patient R., 52 years old. a, b — DVI mode; c, d — Flair mode.
Rice. 3. MRI of the brain of patient R., 52 years old. (end) d, f - T2 mode; g, h — T1 mode. Tomograms are arranged sequentially in DVI, Flair, T2- and T1-weighted image modes.
PCR study of the CSF for the JC virus is positive.
Diagnosis: IB+ No. 131988 dated 11/23/16. Focal brain lesion (PML).
Symptomatic treatment was carried out: 25% solution of magnesium sulfate 10.0 ml + 0.9% solution of sodium chloride - 200.0 ml intravenous drip; heparin 5000 units 3 times a day subcutaneously; recognan (citicoline) 1000 mg IV; Cortexin (polypeptides of the cerebral cortex of cattle) 10 mg IM; bisoprolol 5 mg in the morning; enalapril 5 mg 2 times a day; atorvastatin 20 mg in the evening; aspirin 125 mg in the evening; omez (omeprazole) 20 mg 2 times a day; diacarb (acetazolamide) 500 mg. In order to prevent hypodynamic and hypostatic disorders and expand the motor regime, a complex of physical therapy was prescribed.
Against the background of the therapy, the condition is showing positive dynamics. Blood pressure levels were stabilized and antihypertensive therapy was adjusted. The focal neurological deficit partially regressed. Consciousness is clear. Speech disorders (amnestic-semantic aphasia, afferent motor aphasia, mixed agnosia, dyscalculia, dysarthria component) remain, the severity has decreased slightly. Facial expressions are symmetrical, there are no paresis of the limbs.
The patient had a typical vascular history, which most likely made it difficult to make a correct diagnosis. The positive effect of vascular therapy was short-term, but at the same time it made it possible to stabilize the patient’s condition before transfer to a specialized medical facility.
Discussion of the therapy performed
PML is a fairly common disease in patients with reduced immune status. The diagnosis is usually made on the basis of a typical clinical picture, MRI findings and a positive PCR test for JC virus in the CSF. Treatment has not been fully developed and is symptomatic.
Blocking excess CSF production is a pathogenetic approach to deciding on the treatment of a patient with neurological symptoms against the background of liquorodynamic disorders that were present in the patients. Given the presence of manifestations of intracranial hypertension, acetazolamide was used, a unique systemic carbonic anhydrase inhibitor, the only drug that blocks excess cerebrospinal fluid production [20]. The drug was historically classified as a diuretic, but, according to the instructions, the diuretic effect of acetazolamide is weak, and after 3 days it no longer has diuretic properties. The indication for use is intracranial hypertension. Acetazolamide allows long-term compensation of liquor circulation disorders. It should be remembered that correction of liquorodynamic disorders should not be episodic; long breaks in treatment are undesirable. Acetazolamide therapy fulfills all of these conditions in patients with PML. The duration of action of the drug is up to 12 hours. The recommended dosage for patients with intracranial hypertension is 1-3 tablets per day. There is no need for intervals in therapy2.
With encephalopathy of any origin, damage to the neurovascular unit occurs with the development of oxidative stress, to combat which ethylmethylhydroxypyridine succinate was used. This drug is an antioxidant that has antihypoxic, membrane-protective, nootropic, anticonvulsant and anxiolytic effects [21]. Ethylmethylhydroxypyridine succinates are approved for wide medical use for the treatment of stroke, encephalopathy of various origins (hypertensive, atherosclerotic, traumatic, etc.), neurotic and neurosis-like disorders with anxiety, for the relief of withdrawal syndrome in alcoholism, for the treatment of acute intoxication with neuroleptics and a number of others diseases. Derivatives of succinic acid have a pronounced stress-protective effect, which is manifested in the normalization of post-stress behavior, somatovegetative disorders, restoration of sleep-wake cycles, impaired learning and memory processes, and a decrease in dystrophic changes in various brain structures. The drugs are included in the Federal Guidelines for the Use of Medicines. A significant advantage of succinates is that they have few side effects and low toxicity. In patients with minimally expressed manifestations of cerebrovascular pathology, courses of the drug at a dose of 100 mg IM for 2 weeks are recommended as preventive therapy; the regimen can be repeated once every 6 months. It should be especially noted that these doses do not affect systemic hemodynamics, do not require adjustment of antihypertensive drugs, and are compatible with almost all drugs.
Cortexin (polypeptides of the cerebral cortex of livestock) is a drug with proven effectiveness at the clinical, biological, cellular, genetic and molecular levels [22]. It affects all stages of the pathological chain of molecular events leading to neuronal death. The central link in the pathological processes occurring in the brain against the background of hypoxia is a decrease in the ATP content in brain neurons. A decrease in synthesis and an increase in ATP consumption has been proven immediately after stimulation of glutamate receptors with toxic concentrations of glutamate. It is known that a decrease in ATP concentration in neurons during hyperstimulation of glutamate receptors can disrupt the intra- and intercellular signaling system in brain neurons, in particular ion homeostasis, the activity of glycolytic enzymes and oxidative phosphorylation, Ca2+ uptake by mitochondria and protein synthesis. These processes may underlie the death of neurons after hypoxia and the toxic effect of glutamate excessively present in the synaptic cleft. Cortexin is able to restore ATP content after exposure to toxic concentrations of glutamate in young and old neurons. The drug's peptides have a direct and indirect neurotrophic effect on cells, stimulating the growth of neurites or reducing the death of neurons cultured in a medium devoid of growth factors. The main mechanisms of this effect are probably based on changes in the expression of genes that regulate the synthesis of intrinsic neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF). The drug has multiple effects, including cascade regulation of apoptosis, expression of neurotrophic factors, activation of the energy supply of the nerve cell and mitochondrial potential, improves the functioning of glutamate receptors and regulates Ca concentration in the cell. Through a system of anti-inflammatory cytokines, cortexin improves the neurotrophic supply of nerve fibers and reduces autoimmune aggression, promoting the restoration and growth of axons. It is assumed that the positive effect of cortexin is explained not only by the action of polypeptide components, but also by the neurochemical activity of macro- and microelements, as well as vitamins (A, E, B1 and PP) [22]. Unlike many nootropic drugs, Cortexin has a stimulating effect on mental functions, and in some cases corrects abnormal bioelectrical activity of the brain (confirmed by psychological tests and EEG). The drug is also distinguished by the almost complete absence of adverse reactions and extremely favorable tolerability by patients of any age [22]. In 2009, The Open Neuropsychopharmacology Journal published a study on the use of the drug Cortexin and its effect on cognitive functions and behavioral reactions (under experimental conditions) [23]. Recently, new data have emerged on the indirect immunostimulatory effect of cortexin [24]. The balance of the drug’s peptides and the versatility of its subtle mechanisms of action explain not only the therapeutic effectiveness, but also the absence of side effects of the drug. The course of treatment ranges from 10 to 20 days.
However, if patients have severe neurological symptoms (acute stroke-like episodes with general cerebral and focal symptoms, cognitive impairment, episodes of dizziness or sudden falling, etc.) due to the use of chemically synthesized, foreign drugs at this stage, it is recommended to add other drugs. At the same time, natural metabolites of biochemical processes in the body make it possible, already in the early stages of the disease, to reduce the progression of the process and prevent further neuron degeneration. Priority is given to the administration of drugs that affect phospholipids and the products of their metabolism - since phospholipids are the main structural component of all cell membranes, numerous cell functions directly depend on them. In addition to phospholipids and cholesterol, various proteins are also built into the membrane, which are receptors for hormones, enzymes, and biologically active substances. The normal functioning of proteins, including peptide drugs, directly depends on the phospholipids surrounding them. From this point of view, research data on the use of citicoline are of undoubted interest. Recognan (citicoline) is a drug that has been well studied and widely used for various forms of cerebrovascular pathology in Western Europe, the USA and Japan over the past 30 years [25]. Citicoline is a natural metabolite of biochemical processes in the body, i.e. it is not a foreign chemical compound, a xenobiotic, like most drugs. It contains cytidine and choline linked by a diphosphate bridge and is a necessary intermediate in the synthesis of phosphatidylcholine, the major brain phospholipid, in the phospholipid synthesis pathway (Kennedy pathway). Citicoline reduces the loss of phosphatidylcholine, which is part of the cell membrane, is involved in the synthesis of the neurotransmitter acetylcholine, stimulates the activity of tyrosine hydroxylase and the secretion of dopamine. In experiments, citicoline reduced neuronal degeneration in the hippocampus of rats caused by injection of beta-amyloid protein [26]. Amyloid beta is a normal protein in the body. The degree of cognitive impairment is directly proportional to its accumulation. Choline in combination with cytidine stimulates the secretion of normal neurotrophic amyloid precursor protein by rat brain cells. Citicoline is able to reduce beta-amyloid deposition in the brain, which is clinically manifested in improving integral indicators of cognitive function. In addition to its effects on beta-amyloid, the neuroprotective effect of citicoline is likely due to the redistribution of the main glutamate transporter EAAT2 into lipid raft microdomains, which leads to increased glutamate uptake. Given these data, many clinical studies have been conducted to evaluate the effectiveness of citicoline in the treatment of cognitive disorders associated with brain aging, cerebrovascular diseases and dementia [27]. European clinical studies found that administration of the drug in different doses for different durations of treatment more significantly improved neurological functions and contributed to early recovery of motor and cognitive functions [28]. In the treatment of patients with PML, citicoline plays another important role: it has an immunomodulatory effect, which is based on a decrease in plasma levels of histamine, while simultaneously increasing TNF-α [29]. The course of treatment is long (up to 6-12 months).
Conclusion
PML is a disease that occurs in patients with various pathologies, including neuroinfections, rheumatoid arthritis, when using certain medications, so it is extremely important for a clinician to know the diagnostic criteria of this nosology, be able to make a correct diagnosis, and begin symptomatic therapy as early as possible. This will improve the patient's condition, given that there is still no effective treatment for PML. Until now, antiviral drugs, cytostatics, serotonin receptor antagonists, and plasmapheresis have been widely used. However, it should be noted that all of the above methods are possible only after the causes of PML have been established, while the patient needs emergency care. Therefore, it is advisable to use pathogenetic therapy regimens using drugs that have pleiotropic effects.
The authors declare no conflict of interest.
Letter of the Federal Service for Surveillance in Healthcare dated February 7, 2014 No. 02I-110/14 “On new data on the safety of the drug Benlysta.” . https://www.garant.ru/products/ipo/prime/doc/70485650/#ixzz3OWJlGxDT
Instructions for medical use of the drug Diacarb.
Discircular encephalopathy
The chronic progressive course of cerebrovascular pathology is accompanied by diffuse multifocal changes leading to hemiparesis, ischemic stroke, and multiple neuropsychological and neurological disorders.
The progression of dyscirculatory encephalopathy is associated with tissue degeneration and the accumulation of aggressive metabolites.
Before using neuroimaging methods, experts attributed most of the causes of cognitive disorders to dyscirculatory encephalopathy. Practice shows overdiagnosis of nosology cases. Nuclear magnetic resonance indicates only a 20% incidence of white matter lesions in elderly patients with vascular disease.
The main difference between the discircular type and a stroke is that it affects not large cerebral arteries, but small penetrating vessels, arterioles. Diffuse damage to small branches causes a number of morphological changes:
- Numerous heart attacks (lacunar);
- Diffuse destruction of white matter;
- Mixed form.
Early detection of any category prevents progression after proper supportive care is given.
Therapy
Leukoencephalopathy is an incurable disease. But be sure to go to the hospital to choose drug addiction treatment. The goal of therapy is to slow the progression of the disease and activate brain function.
Treatment of leukoencephalopathy is complex, symptomatic and etiotropic. In each case it is selected individually.
Your doctor may prescribe the following medications:
- drugs that improve cerebral circulation (Vinpocetine, Actovegin, Trental);
- neurometabolic stimulants (Phesam, Pantocalcin, Lucetam, Cerebrolysin);
- angioprotectors (Stugeron, Curantil, Zilt);
- multivitamins, including B vitamins, retinol and tocopherol;
- adaptogens such as aloe extract, vit;
- glucocorticosteroids that help reduce inflammation (prednisolone, dexamethasone);
- antidepressants (fluoxetine);
- anticoagulants that reduce the risk of thrombosis (Heparin, Warfarin);
- if the disease is viral in nature, Zovirax, Cycloferon, Viferon are prescribed.
- physiotherapy;
- reflexology;
- acupuncture;
- breathing exercises;
- homeopathy;
- phytotherapy;
- massage of the collar area;
- manual therapy.
The difficulty of therapy is that many antiviral and anti-inflammatory drugs do not cross the BBB and, therefore, do not target pathological lesions.
Features of periventricular and residual leukoencephalopathy in children
Chronic lack of oxygen supply and prolonged ischemia of brain tissue leads to damage to subcortical structures, hemispheres, and the brain stem. Pathological foci are found deep in the gray matter and are accompanied by changes in subcortical fibers.
Periventricular encephalopathy is characterized by the predominant localization of pathological foci around the ventricles of the brain.
The residual appearance has congenital and acquired causes. The provoking factor in a child is traumatic injuries to the skull, inflammatory processes inside the skull. A separate type, encephalomyelopathy, occurs due to abnormalities in the structure of the vascular network of the brain.
Symptoms of residual encephalopathy in children:
- Cerebral paralysis;
- Oligophrenia;
- Epilepsy;
- Vegetative-vascular dystonia;
- Restless sleep.
Practice shows the presence of a latent course of nosology in newborns weighing about four kilograms. Clinical symptoms appear after the onset of active blood supply. Cases of dementia in preschoolers and schoolchildren are associated with injuries to the skull.
How long do people live with encephalopathy?
Life expectancy is determined by the clinical form of the disease, the rate of progression, and individual changes in the human body.
Progressive multifocal encephalopathy is accompanied by death 1-3 years after detection. Maintenance therapy increases survival.
Varieties of vascular origin have chronic progression. People with this variety, with proper treatment, live for decades. Reduces the duration of hypertension, pronounced ischemic foci of the brain structure, hemorrhages inside the brain.
Causes
Vascular encephalopathy of the brain occurs as a result of chronic ischemia of brain structures caused by the following factors:
- atherosclerosis;
- essential hypertension;
- symptomatic arterial hypertension in nephrological and endocrine diseases;
- vertebral artery syndrome with osteochondrosis, vascular development anomalies, injuries and degenerative processes in the area of the cervical vertebrae;
- diabetic angiopathy in diabetes mellitus with insufficient glucose control;
- traumatic brain injuries;
- persistent arterial hypotension;
- some arrhythmias;
- systemic vasculitis.
Regardless of the provoking disease, circulatory failure causes hypoxia and lack of nutrients. Brain cells die. In their place, small-focal “silent infarctions” or rarefaction zones are formed. Due to the characteristics of the blood supply, subcortical structures and deep areas of the brain are usually affected first. This causes the “disconnection phenomenon” that underlies most manifestations.